Research
Advanced Dating Techniques Reveal Antarctic Peninsula’s Geological History
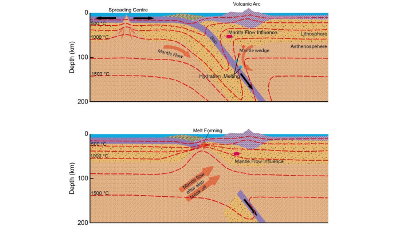
The thesis "Has the formation of a slab window below the Antarctic Peninsula controlled its topographic evolution?" broadly investigates the spatial...
Advancing Creative Circular Economies for Plastics via Technological-Social Transitions (ACCEPT Transitions)
Herein we studied the kinetic modelling of the pyrolysis process for one of the most common plastic wastes, such as polyethylene terephthalate (PET)...
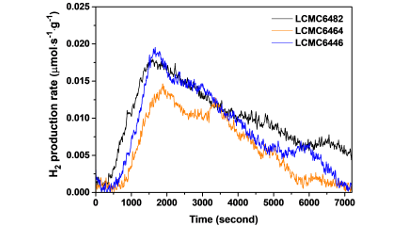
Thermochemical Water Splitting: Boosting Hydrogen Production with Ca-Doped Perovskites”
The study focuses on the development of perovskite oxide structured catalysts for a two-step thermochemical water splitting method that enables the...
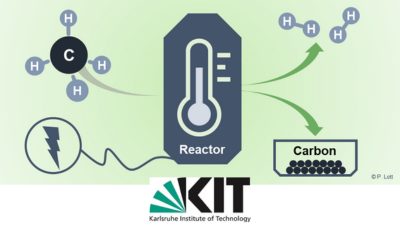
Hydrogen Production and Carbon Capture by Gas-Phase Methane Pyrolysis
In order to reduce carbon dioxide (CO2) emissions, fossil fuels such as oil and natural gas must be replaced by climate-neutral alternatives in the...
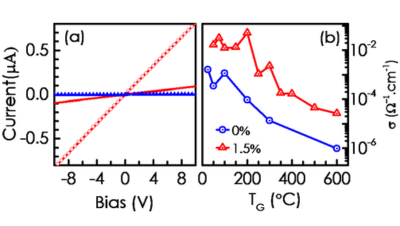
Lithium in NiO Layers on Sapphire: Pulsed Laser Deposition and SIMS Analysis
Wide bandgap oxide semiconductors have tremendous potential for high temperature, high power, high frequency electronics as well as for ultra-violet...
Dr. Toyoda of Nagoya University receives Award!
Dr. Toyoda of Nagoya University has received an award for his paper "Mass spectroscopic measurement of time-varying ion composition in a...
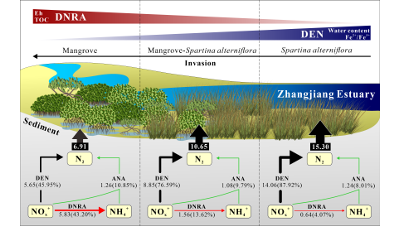
Spartina Invasion Boosts Sediment N-Loss, Lowers N Retention in Mangroves
Wide and rapid Spartina alterniflora invasion has threatened the sustainability of coastal wetlands of China, and has a significant impact on...
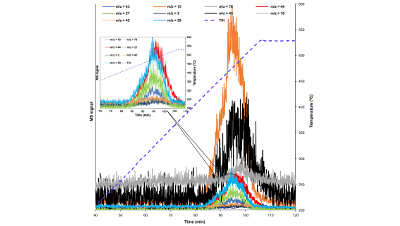
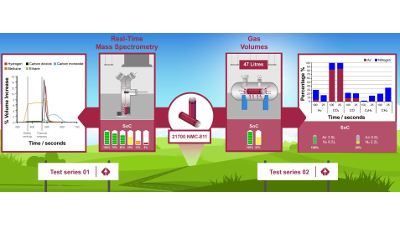
Real-Time Gas Analysis of Li-ion Battery Failure
The failure of lithium-ion batteries can be partly defined by the release of toxic and flammable gases. The composition and volume of these gases...
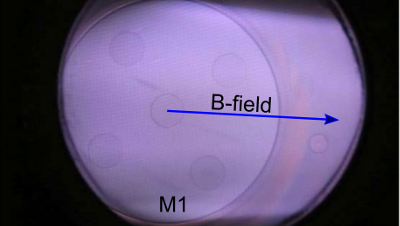
RF plasma sputtering in an ITER-relevant first mirror unit
Metallic first mirrors (FMs) make up important components in many of the optical diagnostic systems in the fusion reactor ITER. They are responsible...