A vast number of products and materials, including high-strength polymers, fuels, refrigerants and cancer treatment drugs, would simply not be possible without the existence of catalysts.1
Catalysts are substances which can increase the rate at which a chemical reaction progresses without being consumed by the reaction. They’re a vital part of the chemical industry – by some estimates, around 90% of all chemical products are catalysed.2
What are heterogeneous catalysts?
Catalysts can be broadly divided into two categories: homogeneous catalysts and heterogeneous catalysts. Homogeneous catalysts are so named because they are dispersed in the same phase as the reactant: for example, a liquid catalyst catalysing liquid reactants. Heterogeneous catalysts, on the other hand, exist in a different phase.
Heterogeneous catalysts are typically solids that catalyse reactions between gaseous reactants. One of the most widespread examples of a heterogeneous catalyst is in the Haber process, the primary industrial process for ammonia production. In this process, nitrogen is reacted with hydrogen at high temperatures and pressures over a solid catalyst (typically iron). Nitrogen undergoes dissociative adsorption on contact with the heterogeneous catalyst, lowering the activation energy required to react with hydrogen, hugely increasing the rate at which ammonia is produced.
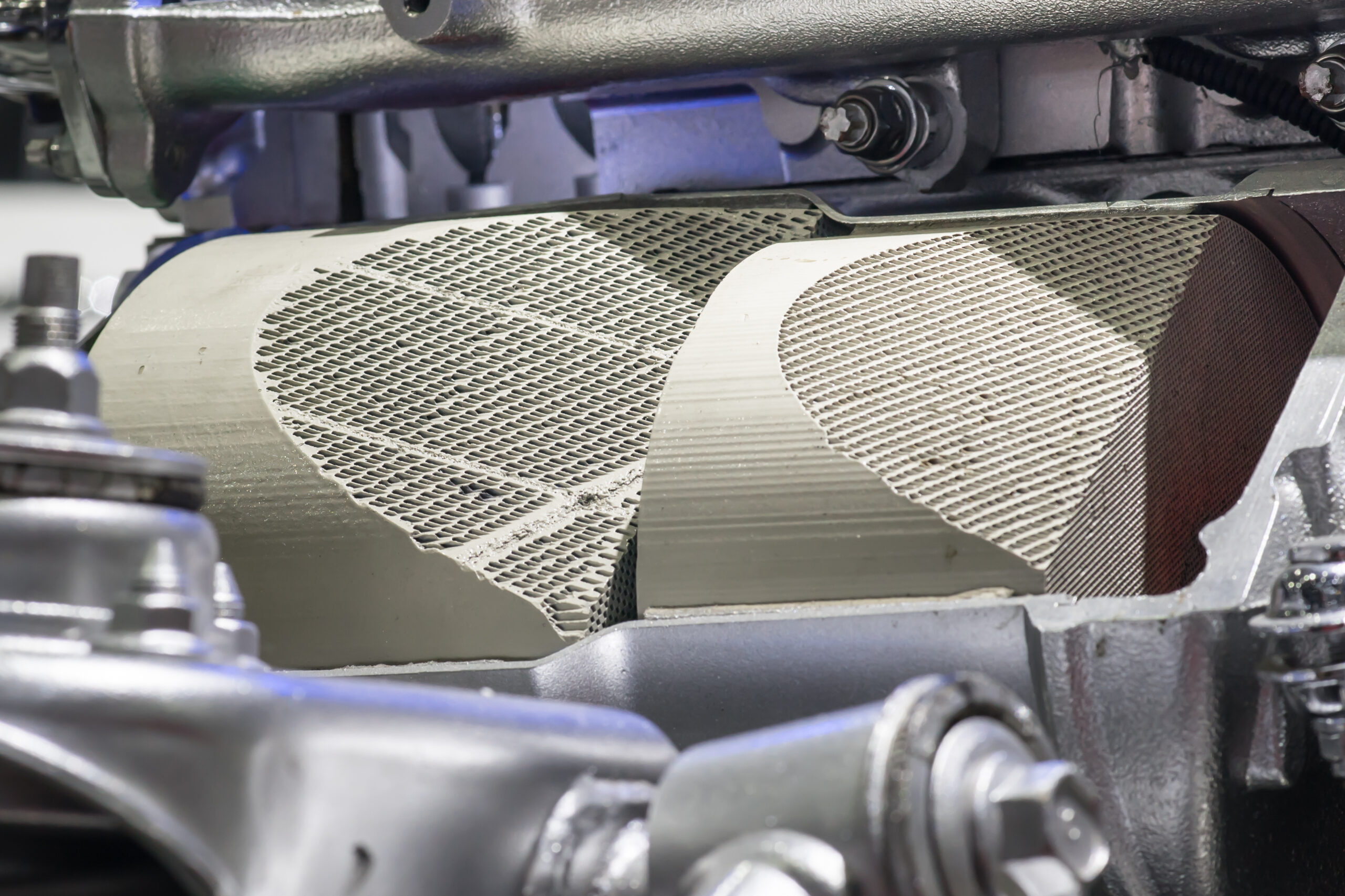
What’s more, because the heterogeneous catalyst is a solid and the reactants (and products) are gases; the catalyst can be used virtually indefinitely. Homogeneous catalysts, by contrast, must be separated out of a mixture once a reaction is finished, which is usually impractical.3
The Haber process illustrates one of the features that make heterogeneous catalysts so useful: heterogeneous catalysts are typically trivially easy to reuse. This means that heterogeneous catalysts are strongly aligned with the “Green Chemistry” relating to efficiency and safety.4
Heterogeneous catalysts and sustainability
Fundamentally, catalysts enable chemical reactions to be carried out with greater energy efficiency. While this is typically motivated by economic concerns rather than environmental ones, heterogeneous catalysts nonetheless play an important role in reducing the energy intensiveness and therefore carbon footprint of a vast array of commercial chemical processes.
Easy reclamation and recycling mean that heterogeneous catalysts have particularly good “green” credentials; offering an improvement in sustainability compared to homogeneous catalysts.
In addition to reducing energy consumption and being easily recyclable, heterogeneous catalysts are playing active roles in improving sustainability in the modern world. One commonplace example of this is the catalytic converter: found in all modern fossil-fuel-powered vehicles, catalytic converters use heterogeneous catalysts (in the form of platinum group metals) to convert toxic gases (like carbon monoxide and NOx species) and particulates into less-toxic substances such as carbon dioxide, water and nitrogen gas.
Heterogeneous catalysts are currently being developed and applied in a range of other sustainable technologies.5 These applications of heterogeneous catalysts include aiding water electrolysis for the production of hydrogen gas (an energy-dense medium for renewable energy storage); and the capture and sequestration of carbon dioxide.
Developing and characterizing heterogeneous catalysts
To develop effective heterogeneous catalysts for new and more sustainable applications, effective catalyst characterization is vital. While understanding reaction kinetics is important, complete characterization involves considering physical properties including total surface area and catalyst dispersion.
Hiden Analytical offer a full range of gas analysis and microreactor systems suitable for comprehensive analysis of heterogeneous catalysts; including systems for pulse chemisorption and temperature-programmed desorption, reduction and oxidation. To find out more, get in touch with a member of the Hiden Analytical team today.
References and Further Reading
1. Bell, A. T. The impact of nanoscience on heterogeneous catalysis. Journal Name: Science; Journal Volume: 299; Journal Issue: 5613; Other Information: Journal Publication Date: March 14, 2003 https://digital.library.unt.edu/ark:/67531/metadc780930/ (2003).
2. Catalysis. https://www.chem.ox.ac.uk/catalysis.
3. Shende, V. S., Saptal, V. B. & Bhanage, B. M. Recent Advances Utilized in the Recycling of Homogeneous Catalysis. The Chemical Record 19, 2022–2043 (2019).
4. Anastas, P. & Nicolas Eghbali. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 39, 301–312 (2010).
5. Hu, X. & Yip, A. C. K. Heterogeneous Catalysis: Enabling a Sustainable Future. Frontiers in Catalysis 1, (2021).

