During the growth of oxide thin films by pulsed laser deposition, there is always the question from which source oxygen is incorporated in the as-grown thin film: Target, background or substrate. This is an essential question since the optimization of the deposition conditions will depend on it. Using 18O exchanged SrTiO3 and LaAlO3 as substrates, a strong oxygen substrate‑to‑film transfer has been experimentally observed for epitaxially grown SrTiO3 and LaAlO3 thin films by SIMS depth profiling. This oxygen transfer effect can seriously change the respective thin film properties.
The preparation of high quality complex oxides thin films and heterostructures with atomic precision has considerably advanced in recent years considerably with a crystalline quality comparable to semiconductors and oxide semiconductors structures. The challenge associated with growing oxides is to provide and control the amount of oxygen during the growth to obtain the desired oxygen stoichiometry and hence appropriate physical properties of the respective oxide. For a deposition using pulsed laser deposition (PLD) it often seems to be sufficient to create the correct amount of plasma species within an oxygen background to form the chosen compound as a thin film on a suitable substrate, and to supply the missing oxygen with a subsequent annealing step.
To investigate the role of oxygen supplied by the substrate during a deposition, the oxygen diffusion properties of SrTiO3 and LaAlO3 thin films grown on 18O isotope exchanged SrTi18O3 and LaAl18O3 substrates were studied by dynamic secondary ion mass spectrometry (D-SIMS) which yields elemental depth profiles. SrTiO3 and LaAlO3 thin films have been prepared at three different deposition temperatures (nominal room temperature, 650°C and 750°C) at a background pressure p=1.5×10-5 mbar and a laser fluence F=4 Jcm-2. SIMS spectra were recorded using a Hiden Analytical EQS quadrupole mass spectrometer operated with a 2.5 keV Ar ion beam focused to 150 µm diameter rastering over a square of 1x1mm with an effective sampling area of 500×500 µm. The etched area is subsequently measured with a Dektak 8 profilometer to convert etching time into depth. (see Fig. 1). In addition a kinetic energy selection scheme is used to separate species with the same mass.
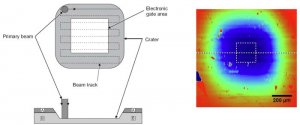
Figure 1: Schematic representation of the raster crater (left) and a real crater measured with a profilometer (right). The different colours correspond to different depth levels.
The 18O diffusion from SrTi18O3 into SrTiO3 shows a pronounced dependence on the deposition temperature (Fig. 2a). Whereas for a room temperature deposition, no traceable 18O diffusion into the film has been measured, the situation changes dramatically for elevated deposition temperatures. At 650°C, there is considerable 18O diffusion from the substrate into the film, while at TS=750°C there is no significant difference with respect to the amount of 18O measured in the film and substrate. In the case of SrTiO3 grown on LaAl18O3 a significant and homogeneous oxygen contribution is measured for the SrTiO3film prepared at TS=750°C (Fig. 2b). Even at 650°C, there is still a significant 18O intake which can be detected up to the film surface. Measuring Sr and Ti species from the substrate and film simultaneously with 16O and 18O, the elemental composition of film and substrate are very similar. This implies that oxygen in the SrTiO3 thin film is supplied by the substrate and the oxygen provided by the target seems to play a minor role for this system. The results also indicate that the initially formed film is oxygen deficient and a chemical gradient is in favour of supplying oxygen via the substrate.
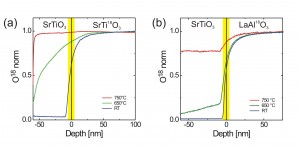
Figure 2: a) 18O SIMS depth profile of SrTiO3 on SrTi18O3 grown at Ts=750°C, 650°C and room temperature. The sharp drop of the 18O signal near the SrTiO3surface for the film grown at TS=750°C could be related to a back-exchange of 16O at room temperature. b) 18O SIMS depth profile of SrTiO3 on LaAl18O3grown at Ts=750°C, 650°C and room temperature.
Paper Reference: C. W. Schneider, M. Esposito, I. Marozau, K. Conder, M. Doebeli, Yi Hu, M. Mallepell, A. Wokaun, and T. Lippert (2010) “The origin of oxygen in oxide thin films: Role of the substrate” Appl. Phys. Lett. [online] http://dx.doi.org/10.1063/1.3515849
Hiden Product: EQS Secondary Ion Mass Spectrometer Bolt on SIMS Analyser
Read Full Article: Customer Research Review Issue 1120/09 Thin Films, Plasma and Surface Engineering

