Research and development (R&D) into novel energy storage materials is important to improve the utility of energy generated from renewable sources, thereby reducing reliance on fossil fuels, a finite resource. Lithium ion batteries are a central to such initiatives, and are increasingly pivotal in green markets like that of hybrid electric vehicles (HEVs).
Fossil fuels are a finite resource which puts unique pressures on research and development (R&D) into novel energy storage materials for renewables. Li-ion batteries are key to these initiatives, subsequently representing one of the most promising avenues for clean energy systems.
The Basics of Li-Ion Batteries
There are three components of a lithium ion battery: the cathode, the anode, and the electrolyte. Cathodes are usually the most expensive and heaviest element of the battery meaning that cathode studies are high priority. The cathode is the oxidizing or positive electrode that obtains electrons from the external circuit and is diminished throughout the electrochemical reaction.
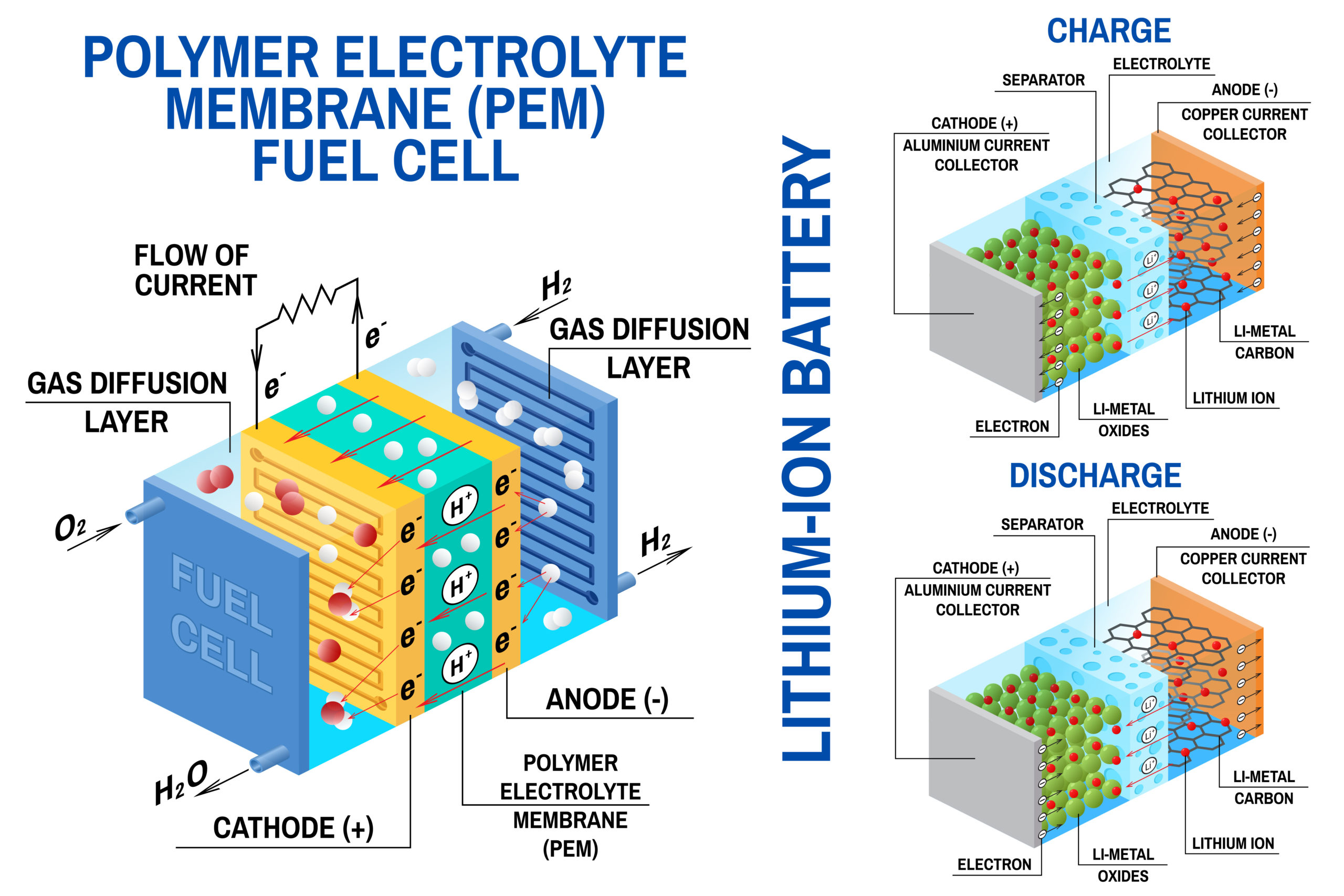
Cathodes are often made from lithium cobalt oxide (or lithium cobaltate), lithium manganese oxide (also known as spinel or lithium manganate), lithium iron phosphate, as well as lithium nickel manganese cobalt (or NMC) and lithium nickel cobalt aluminum oxide (or NCA). As the unifying element in each of these li-ion battery types, lithium is naturally central to the essential reactivity of cathodes.
Understanding Cathode Studies
Cathode studies usually focus on the discovery and exploration of new and improved materials which can be used for energy storage applications. The materials which are used have a significant impact on the performance of the battery. In disposable batteries, electrodes can flow in a single direction from anode to cathode. However, rechargeable batteries have to reverse the flow. Cathode studies led to the identification of lithium as an ideal substance for both anode and cathode as it accepts and loses electrons respective to the configuration of the electrode element.
Cathode studies have focused on materials which can facilitate both discharge and recharge cycles across variable ion and electron transportation and are now an area of concentrated research. Cathode studies are currently investigating numerous structural groups for the future of energy storage devices, primarily using differential electrochemical mass spectrometry (DEMS) as a key research tool. The electrochemical performances of different li-ion battery systems depends on distinct chemistries, crystal structures, and solid-state physics.
DEMS is an ideal research tool for cathode studies as it combines electrochemical half-cell experimentation with precision mass spectrometry. This allows researchers to accurately determine low level reaction intermediates occurring as a result of reactions at the interfaces between cathodes and electrolytes.
Hiden Analytical for Cathode Studies
Hiden Analytical is at the forefront of differential electrochemical mass spectroscopy solutions for cathode studies and lithium-ion battery research. Cathode studies are an important area of research and expansion for sustainable energy storage devices which are lightweight enough for a range of mobile electronics applications.
Our DEMS solution combines the powerful HPR-40 DSA mass spectrometer with a unique nanoporous sampling interface that allows for real-time detection of both evolved off-gas and dissolved species in solution. It is compatible with a large number of cathodes and anodes and contribute to a broad scope of research. Contact us today to find out more.

