The electrochemical CO2 reduction reaction (CO2RR) powered by renewable energy to produce valuable chemicals and fuels has become a promising strategy for using renewable energy to produce fuels and chemicals. The process can occur under ambient temperature and normal pressure conditions. This approach potentially aids in mitigating the climate change problem by initiating a carbon-neutral cycle. However, it is a significant challenge to obtain mechanistic information about the CO2RR, as this is complicated by the multiple possible proton-electron transfer pathways and associated intermediates, and also accompanied by the competing hydrogen evolution reaction (HER). It is widely reported that the CO2RR performance (such as activity and selectivity) is strongly dependent on the nature and structure of catalysts and electrolytes. High-resolution correlative characterization plays a central role in interrogating the complex electrochemical reactions that involve multiple pathways and various products.
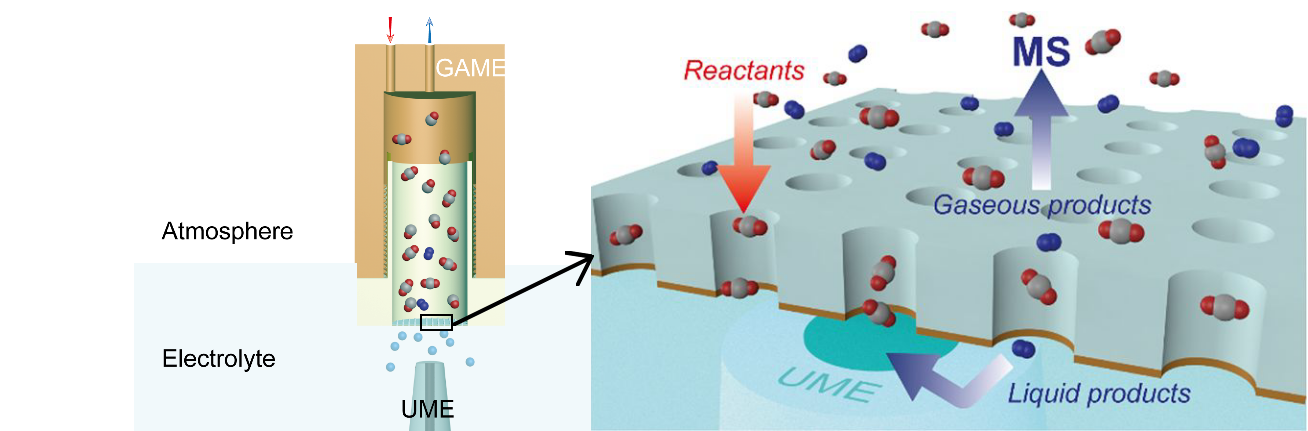
Figure 1. Game electrode. Lefthand side showing hollow structure of electrode leading to efficient collection of product species at the gas/liquid interface.
In relation to the CO2RR, the mechanism and kinetics have not been fully understood, predominantly limited by the unclear electrochemistry-product distribution relationship. Recently, Dr. Guohui Zhang, Youxin Cui and Prof. Anthony Kucernak from Imperial College London have developed tools for real-time and in-situ measurements of the CO2RR. Combining the recently developed “gas accessible membrane electrode” (GAME, figure 1) with mass spectrometry (GAME-MS) using a Hiden Analytical QGA system, the evolution of gaseous products can be tracked synchronously during the electrochemical CO2RR, figure 2.
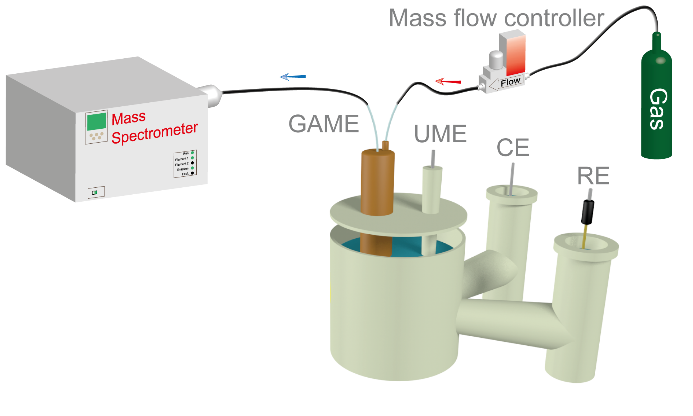
Figure 2. Cartoon of electrochemical MS configuration showing GAME electrode placed in electrochemical cell allowing collection of product gases.
From the synchronized electrochemical-mass spectrometric results, the evolution of diverse CO2RR products (H2, C2H4, CH4) is elucidated, providing insights about the onset potentials for individual products and the Tafel slopes for the overall reaction, figure 3. It should also be highlighted that the estimated Tafel slopes for individual products can be obtained from the MSCVs enabled by the GAME-MS, which are critical to the interpretation of the electrokinetic behaviour. Such information will be useful in revealing the possible reaction pathways leading to these products. These approaches can be extended to study many other electrochemical reactions, providing insights for fundamental understanding and practical applications.
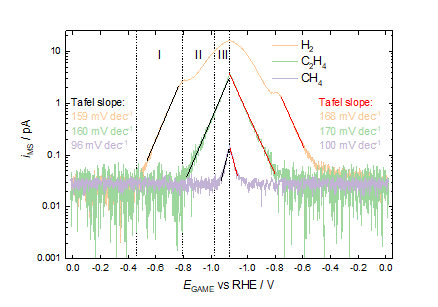
Figure 3. Mass spectrometric detection of product gases during cyclic voltammetry of a nanostructured copper electrode recorded in 0.5 M KHCO3 at a scan rate of 1 mV s-1 using a pure CO2 atmosphere as a carrier gas.
 Project summary by: Anthony Kucernak (Kucernak Group), Department of Chemistry, Imperial College London, London SW7 2AZ, United Kingdom
Project summary by: Anthony Kucernak (Kucernak Group), Department of Chemistry, Imperial College London, London SW7 2AZ, United Kingdom
Paper Reference: “Real-Time In Situ Monitoring of CO2 Electroreduction in the Liquid and Gas Phases by Coupled Mass Spectrometry and Localized Electrochemistry” ACS Catalysis (2022) 12, 10, 6180-6190 doi.org/10.1021/acscatal.2c00609
Hiden Product: QGA
To find out more about these products visit the QGA product page or if you would like to contact us directly please Send us a Message.

