Metal alanates are regarded as a very promising group of materials for high-density solid-state hydrogen storage. Therefore, the search for new alanates with high hydrogen content and sufficiently fast reaction kinetics of absorption and desorption at moderate temperatures is still a very active field.
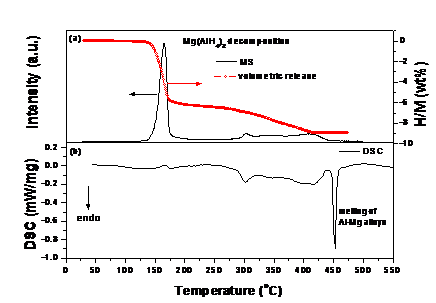
In this work, we developed a modified synthesis method for Mg(AlH4)2 with high purity. By ball milling the mixture NaAlH4 and MgCl2 (molar ratio: 2:1) in Et2O followed by Soxhlet extraction, Mg(AlH4)2 submicron rods were successfully obtained as the resultant product, and its purity was determined to be as high as 96.1%. Upon heating, ~ 9.0 wt% of hydrogen was released from the as-prepared Mg(AlH4)2 with a three-step reaction. This was measured by a combination of MS (m/z 2, Hiden QIC-20) and volumetric analyses. At 125-200ºC, Mg(AlH4)2 decomposed first to liberate hydrogen and generate MgH2 and Al (Figure 1 (a)). With increasing temperature to 320ºC, the newly produced MgH2 reacted with Al to form the Al0.9Mg0.1 solid solution along with hydrogen release. As the temperature was further elevated to 440ºC, the reaction between the Al0.9Mg0.1 solid solution and the remaining MgH2 occurred to evolve additional hydrogen and form Al3Mg2. DSC measurement showed that the first step dehydrogenation is exothermic while the last two steps are endothermic (Figure 1 (b)).
Analysis of the isothermal and non-isothermal behaviours revealed a diffusion-controlled kinetic mechanism for the first step dehydrogenation, and its apparent activation energy was calculated to be about 123.0 kJ/mol. However, only ~2.3 wt% of hydrogen could be recharged at 140-210 °C and 100 bar of hydrogen pressure. Therefore, further improvement on hydrogen storage reversibility of Mg(AlH4)2 should be performed to make it useable as a potential hydrogen storage material.
Project Summary by:
Y. Liu, Y. Pang, M. Gao & H. Pan
State Key Laboratory of Silicon Materials,
Key Laboratory of Advanced Materials & Applications for Batteries of Zhejiang Province &
Department of Materials Science and Engineering, Zhejiang University
Zhejiang Province, Hangzhou 310027 China
Paper Reference: Liu et al. (2012) “Synthesis and hydrogen storage thermodynamics and kinetics of Mg(AlH4)2 submicron rods” International Journal of Hydrogen Energy, 37, (23), 18148-18154