The objective of this study is to compare the performance of several activated carbon (AC) based catalysts for CH4 reforming with CO2, including raw AC and AC modified with HNO3 and NaNO3, designated as AC-HNO3 and AC-NaNO3, respectively. CH4-CO2 reforming experiments were conducted in a fixed-bed reactor at temperatures from 700 to 1,000°C. The catalysts were characterized by various techniques, including Fourier Transform Infrared Spectroscopy (FTIR), Brunauer-Emmett-Teller (BET) analysis, CO2 chemisorption, temperature programmed oxidation-CO2 (TPO-CO2), and scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX). The catalytic activities of the three catalysts are considerably different at low temperature, although they are similar at high temperature. At 700 °C, the average conversions of CH4 and CO2 over AC-NaNO3 are 17.7% and 29.7%, respectively, which are 2.4 and 3.2 times higher than those over AC. At 1,000oC, the conversions of CH4 and CO2 exceed 90% over these three catalysts. In addition, the mole ratio of H2/CO increases with temperature for all the catalysts. The mole ratio of H2/CO over AC-HNO3, which is from 0.76 to 0.94 at different temperatures, is closer to 1 than that over the other two catalysts. Based on the characterization results, NaNO3 is more instrumental to the formation of mesopores, surface oxygenated groups, and the reduction of deposited carbon. Hydroxyl group plays an important role in CH4-CO2 reforming. According to TPO-CO2 observations, two types of deposited carbon are formed during reforming reaction. The apparent activation energies of the CH4-CO2reforming reactions catalyzed by AC, AC-HNO3, and AC-NaNO3 are 202 kJ/mol, 227 kJ/mol, and 123 kJ/mol.
TPO-CO2 of the used catalyst was done using the same TA along with a mass spectrometer (Hiden, HPR-20 TMS). The used catalyst was heated in 100 ml/min CO2 from room temperature to 1,200oC at the rate of 5°C/min, while the generation of CO was measured by the online MS. The electron ionization voltage of the MS was 0-220 eV.
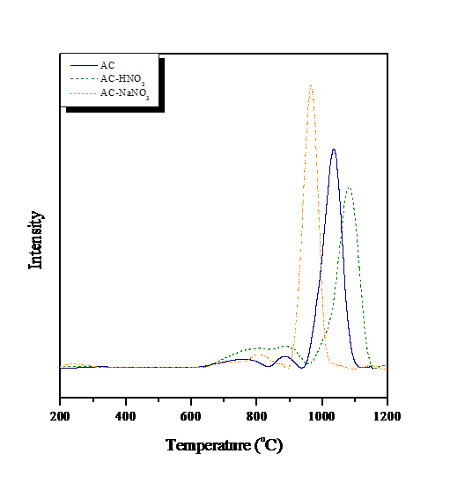
Figure. TPO-CO2 profiles of the used catalysts [Catalyst weight: 0.3 g; Inlet flow rate: 20 ml/min; Inlet CH4:CO2 mole ratio: 1; Temperature: 900oC; Reaction time: 240 min]
Project Summary by: Maohong Fan, Department of Chemical & Petroleum Engineering, University of Wyoming, Laramie, WY 82071 USA

Paper Reference: L. Xu et al. (2014) “Catalytic CH4 reforming with CO2 over activated carbon based catalysts” Applied Catalysis A: General 469, 387-397
Hiden Product: HPR-20 TMS Transient MS
Read Full Article: Customer Research Review : Gas reaction studies & catalysis research [issue : 1120/10]

