 Recently, because of the increasing demand for natural gas and the reduction of greenhouse gases, interests have focused on the production of synthetic natural gas (SNG), which is suggested as an important future energy carrier. Hydrogenation of CO2, the so-called methanation reaction, is a suitable technique for the fixation of CO2. Nickel supported on yttrium oxide and promoted with cobalt were prepared by the wet-impregnation method respectively and characterized using SBET, XRD, FTIR, XPS, TPR, and HRTEM/EDX. CO2 hydrogenation over the Ni/Y2O3 catalyst was examined and compared with Co-Ni/Y2O3 catalysts, Co% = 10 and 15 wt/wt. Carbon dioxide hydrogenation was carried out in a homemade fixed bed tubular reactor operating at atmospheric pressure. The quartz silica reactor was heated in an electric furnace equipped with a programmable temperature controller. 2 gm of the fresh catalyst were diluted with silicon carbide (SiC) to obtain 5cm bed height packed in the middle of the reactor. The temperature was monitored by a K-type thermocouple placed in the center of the catalyst bed. Before the catalytic test, all the samples were activated in situ with a 30 mL/min flow of pure hydrogen at atmospheric pressure for 2 h at 600 °C. After the reduction, the catalysts were cooled down and the flow of premixed gas at a molar ratio CO2/H2/Ar = 1/4/5 with GHSV of 6000 ccg-1h-1 was gradually introduced through the catalysts. Then, the temperature was increased to 350 °C and the reaction time was 3h. Gaseous reaction products were analyzed on-line by a quantitative gas analysis system (HIDEN ANALYTICAL QGA, England). The selectivity of H2, CO2, CH4 and, CO was detected by the gas analyzer using a matrix equation to correct the overlapped detection values from the m/z of 28 (CO), 44 (CO2), 2 (H2), and 16 (CH4).
Recently, because of the increasing demand for natural gas and the reduction of greenhouse gases, interests have focused on the production of synthetic natural gas (SNG), which is suggested as an important future energy carrier. Hydrogenation of CO2, the so-called methanation reaction, is a suitable technique for the fixation of CO2. Nickel supported on yttrium oxide and promoted with cobalt were prepared by the wet-impregnation method respectively and characterized using SBET, XRD, FTIR, XPS, TPR, and HRTEM/EDX. CO2 hydrogenation over the Ni/Y2O3 catalyst was examined and compared with Co-Ni/Y2O3 catalysts, Co% = 10 and 15 wt/wt. Carbon dioxide hydrogenation was carried out in a homemade fixed bed tubular reactor operating at atmospheric pressure. The quartz silica reactor was heated in an electric furnace equipped with a programmable temperature controller. 2 gm of the fresh catalyst were diluted with silicon carbide (SiC) to obtain 5cm bed height packed in the middle of the reactor. The temperature was monitored by a K-type thermocouple placed in the center of the catalyst bed. Before the catalytic test, all the samples were activated in situ with a 30 mL/min flow of pure hydrogen at atmospheric pressure for 2 h at 600 °C. After the reduction, the catalysts were cooled down and the flow of premixed gas at a molar ratio CO2/H2/Ar = 1/4/5 with GHSV of 6000 ccg-1h-1 was gradually introduced through the catalysts. Then, the temperature was increased to 350 °C and the reaction time was 3h. Gaseous reaction products were analyzed on-line by a quantitative gas analysis system (HIDEN ANALYTICAL QGA, England). The selectivity of H2, CO2, CH4 and, CO was detected by the gas analyzer using a matrix equation to correct the overlapped detection values from the m/z of 28 (CO), 44 (CO2), 2 (H2), and 16 (CH4).
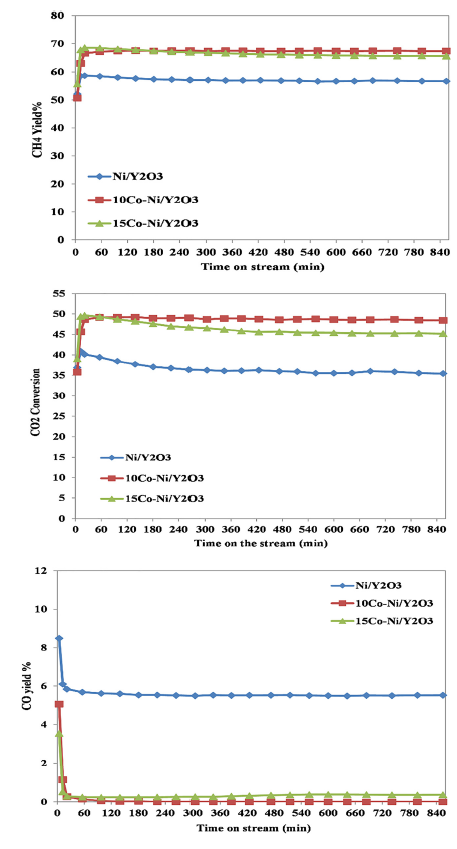
The CH4 yield reached 67% and CO2 conversion extended to 48.5% with CO traces over 10Co-Ni/Y2O3 catalyst. This encourages the direct methanation reaction mechanism. However; the reaction mechanism over Ni/Y2O3 catalyst show different behaviors rather than that over bi-metal catalysts whereas the steam reforming of methane reaction was arisen associated with consumption of methane besides increase in H2 and CO formation; at the same reaction temperature.
Top figure: CO2 conversion, CH4 yield%, and CO yield% during the methanation reaction over yttrium-based catalysts.
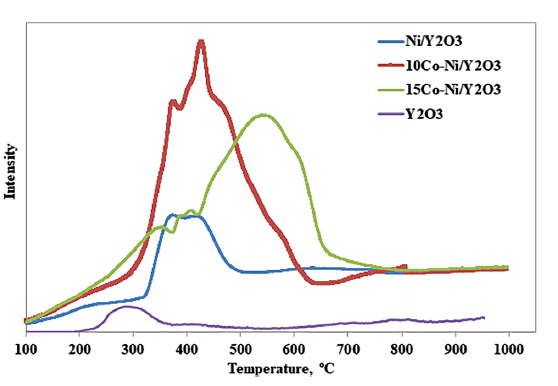
Bottom figure: H2-TPR of the prepared catalysts
 Project summary by: Radwa A. El-Salamony, Egyptian Petroleum Research Institute (EPRI), Cairo, Egypt
Project summary by: Radwa A. El-Salamony, Egyptian Petroleum Research Institute (EPRI), Cairo, Egypt
Paper Reference: “CO2 Valorization into Synthetic Natural Gas (SNG) using a Co-Ni bimetallic Y2O3 Based Catalysts”, Int. J. Chem. React. Eng (2021) 19, 6, 571-583 doi.org/10.1515/ijcre-2020-0163
Hiden Product: QGA
Reference: AP-QGA-202125
To find out more about these products visit the QGA product page or if you would like to contact us directly please Send us a Message.

