 Nitrogen oxide (NOx) causes significant impacts on the environment and human health, and nitrogen dioxide (NO2) is the most toxic and prevalent form of NOx in the atmosphere. Its removal and catalytic degradation into non-harmful species (i.e., N2) are thus important challenges. A great deal of effort has been devoted to developing catalysts for deNOx processes with a focus on NO reduction from exhaust gases. The ammonia-assisted selective catalytic reduction (NH3-SCR) over Cu-exchanged zeolites is by far the most effective method to reduce NOx to N2 and H2O. However, this process has several inherent limitations, notably the high operating temperature (typically 250-550 oC), high running cost for reductants, use of corrosive and toxic NH3, and potential release of NH3 into atmosphere. In contrast, the catalytic degradation of NO2 for domestic environments is poorly studied. The development of new efficient catalysts and catalytic processes to enable the direct decomposition of NO2 at room temperature and without the use of reducing agents has, therefore, attracted considerable attention.
Nitrogen oxide (NOx) causes significant impacts on the environment and human health, and nitrogen dioxide (NO2) is the most toxic and prevalent form of NOx in the atmosphere. Its removal and catalytic degradation into non-harmful species (i.e., N2) are thus important challenges. A great deal of effort has been devoted to developing catalysts for deNOx processes with a focus on NO reduction from exhaust gases. The ammonia-assisted selective catalytic reduction (NH3-SCR) over Cu-exchanged zeolites is by far the most effective method to reduce NOx to N2 and H2O. However, this process has several inherent limitations, notably the high operating temperature (typically 250-550 oC), high running cost for reductants, use of corrosive and toxic NH3, and potential release of NH3 into atmosphere. In contrast, the catalytic degradation of NO2 for domestic environments is poorly studied. The development of new efficient catalysts and catalytic processes to enable the direct decomposition of NO2 at room temperature and without the use of reducing agents has, therefore, attracted considerable attention.
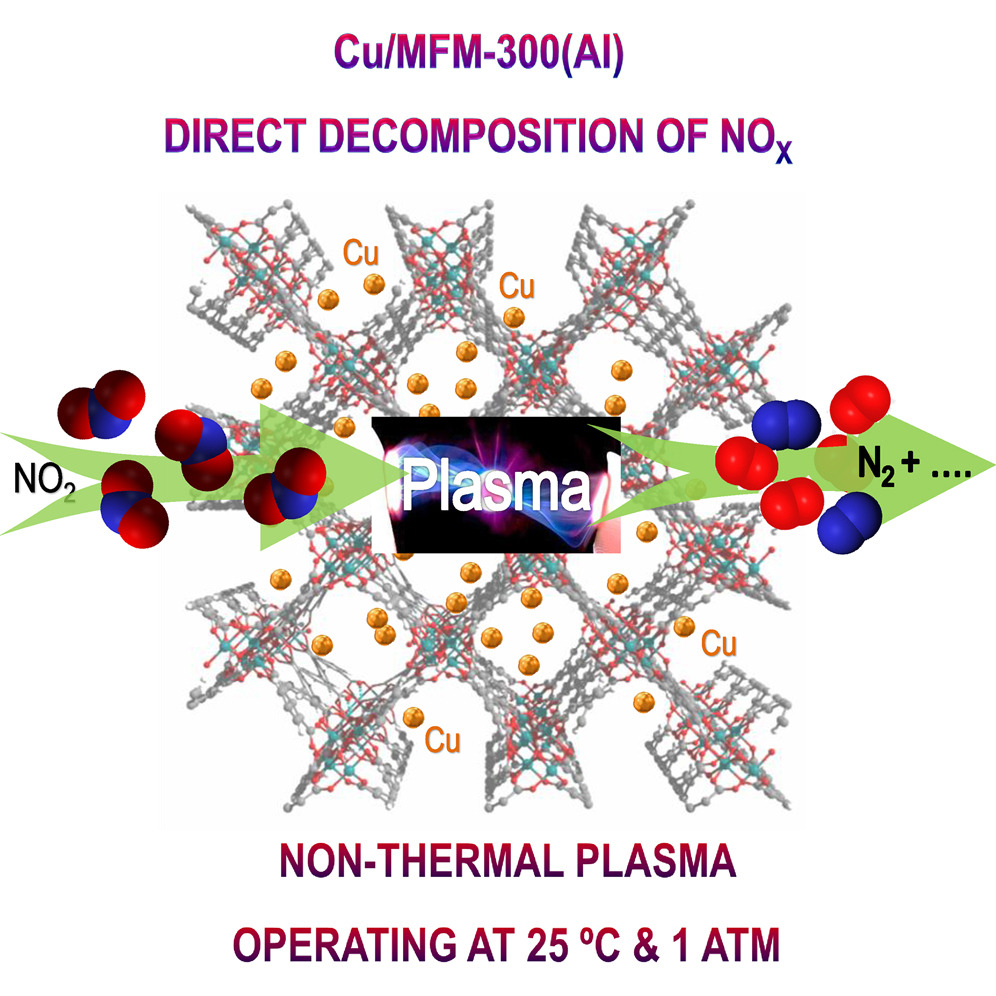
Porous metal-organic framework (MOF) materials show promise for the highly selective adsorption and separation of NO2 at room temperature with the high porosity and tailored-to-design pore functionality. However, the application of MOF-based catalysts in deNOx processes has been rarely explored, primarily due to the limited stability of MOFs against highly corrosive NO2 and NH3 at elevated temperatures. Non-thermal plasma (NTP) activation can promote deNOx processes at room temperature by generating highly reactive species, especially vibrationally and electronically excited states of molecules (e.g., N2, O2, NO), N and O atoms, radicals, and electrons with a typical electron temperature of 104 oC. NTP activation in MOF-based catalysts has been shown to enhance performance in catalytic reactions, with the structure and porosity of the MOF being preserved. There are thus powerful drivers for the development of efficient deNOx systems that can operate at room temperature and avoid the use of toxic reductants.
In this study here, a new process combining a robust MOF-based catalyst and NTP-activation has been developed for one-through conversion of NO2 into N2 without use of any external reducing agent at room temperature. The rigid and robust open structure of MFM-300(Al) offers an excellent platform to embed uniformly dispersed Cu nanoparticles of diameters of ca. 1 nm using a simple incipient wetness impregnation method. Cu/MFM-300(Al) shows simultaneously high NO2 conversion and high N2 selectivity, as well as an excellent long-term stability under the NTP activation at 25 oC and 1.0 bar. The high catalytic activity of Cu/MFM-300(Al) is attributed to the unique formation of Cu2+⋯NO nitrosylic adducts, which facilitates the dissociation of NO to improve the yield of N2. Compared with conventional thermal-based catalysis, NTP activation can effectively preserve the structure and porosity of MOF-based catalysts. Coupled with emerging stable MOFs showing ultra-high and selective NO2 adsorption, we are now seeking to design new MOF-based catalysts to drive future development of efficient reductant-free deNOx processes to mitigate air pollutants.
 Project summary by: Martin Schröder, Department of Chemistry University of Manchester, Manchester M13 9PL, UK
Project summary by: Martin Schröder, Department of Chemistry University of Manchester, Manchester M13 9PL, UK
Paper Reference: “Catalytic decomposition of NO2 over a copper-decorated metal–organic framework by non-thermal plasma” (2021) Cell Reports Physical Science 2 (2), 100349
Hiden Product: QGA
Reference: AP-QGA-202118
To find out more about these products visit the QGA product page or if you would like to contact us directly please Send us a Message.

