Elucidating Acidic Electro-Oxidation Pathways of Furfural on
Platinum
ACS Catalysis 2019, 9, 10305−10316 doi:10.1021/acscatal.9b02656
Biomass is being investigated as a source of hydrocarbon feedstock as a more sustainable alternative to fossil fuels. There has been interest in electrochemical organic synthesis methods to obtain these required chemicals. A key aspect to make the synthesis more efficient is to catalyse the oxidation process and some researchers have been investigating Electrocatalysis as a novel way to achieve this goal. A flow cell allows flow of the electrolyte and reference and counter electrodes could apply potential. Reactant gases were extracted through the cell membrane to the Hiden mass spectrometer for investigation. Relating the potential with the mass spectrometry species allows the reactions to be characterised.
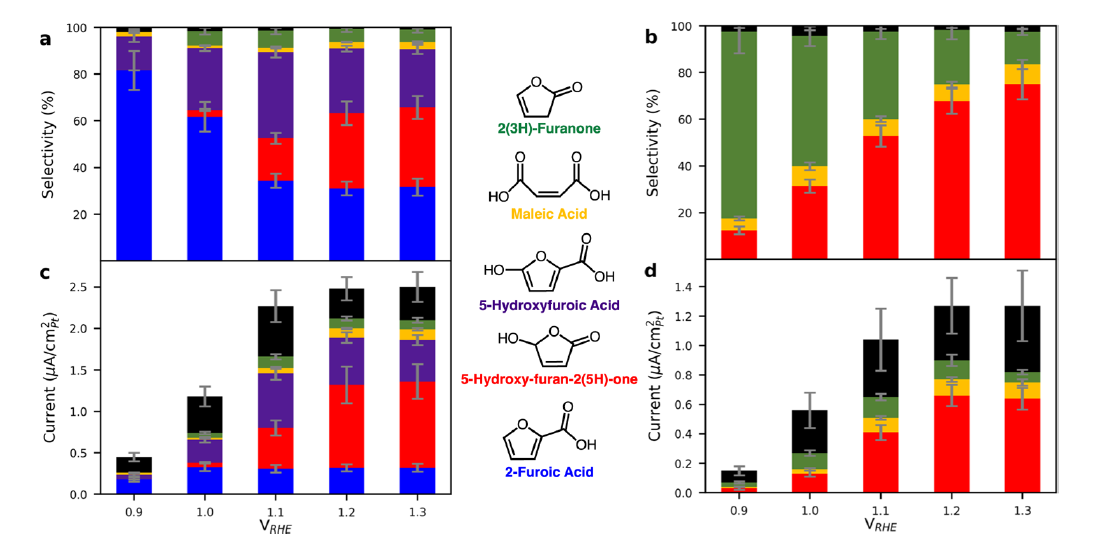 Figure 2. Selectivity and partial currents toward major products of the electro-oxidation of 100 mM furfural (a,c) and 100 mM FA (b,d) on Pt/C in 0.25 M HClO4 at the steady state. FA (blue), 5-hydroxy-2(5H)-furanone (red), HFA (purple), MA (yellow), 2(3H)-furanone (green), and terminal CO2 (black) (CO2 current that is concomitant with C4 products is allocated to those respectively).
Figure 2. Selectivity and partial currents toward major products of the electro-oxidation of 100 mM furfural (a,c) and 100 mM FA (b,d) on Pt/C in 0.25 M HClO4 at the steady state. FA (blue), 5-hydroxy-2(5H)-furanone (red), HFA (purple), MA (yellow), 2(3H)-furanone (green), and terminal CO2 (black) (CO2 current that is concomitant with C4 products is allocated to those respectively).
You can Download & read the full paper or View it online.
To find out more about this product visit the HPR-40 DEMS product page or if you would like to contact us directly please Send us a Message.

