In radiation detection, gaseous detectors have an important role in the characterization of incident radiation. Experiences, namely in X-ray spectrometry, X-ray astronomy, medical instrumentation and high energy physics use gaseous detectors, usually with a rare gas, as a detection medium. Recently it was suggested the addition of molecular additives to the main gas to improve some characteristics of the detection medium. One of this molecular additives suggested was trimethylamine (TMA – (CH3)3N) [1]. However, these mixtures (Xe-TMA) lack in information. Also, information on TMA is scarce. For an example, concerning ionization energy and appearance energy of the different TMA ions there is a void on TMA data.
In this work, we used the quadrupole mass spectrometer, model HAL 200-RC Hiden to assess the ionization energy, appearance energy and the relative abundances of the TMA ions’ as function of the incident electron energy. In order to identify the sequence of ions produced by increasing electron energy in TMA, a technique was devised using the mass spectrometer in which the energy of the incident electrons was varied from 7.5 to 70 eV.
Among all the TMA peaks detected, only the ten more intense were analyzed. These correspond to ~93% of the total number ions formed by electron impact at 70 eV.
The residual gas analyzer was assembled to an ultra-high vacuum system with a rotary pump (DS-302 Varian) and a turbomolecular pump (Turbovac 151 Leybold).
Prior to introducing the gas in the RGA, the gas system was evacuated down to 1×10-7 Torr. The TMA gas used (99% purity), entered the RGA through a needle valve at a constant flow and its pressure was kept at ~8×10-6 Torr during the analysis, with evacuation on. This pressure was checked with a Varian, model 525 cold cathode vacuum gauge.
The electron energy was increased from lowest value studied, 7.5 eV, at 0.1 eV intervals near the inferred thresholds and with higher energy steps for the other ranges. Results have been corrected for the energy distribution of the filament emitted electrons and peak distribution width.
In this work, the value found for the ionization energy, corresponding to the C3H9N+ on set, was 7.9 ± 0.2 eV. The appearance of the second different ion (C3H8N+) was measured at 9.6 ± 0.2 eV. At 15.5 eV electron energy all the ten ions’ studied had already been detected. Up to 18 eV, the more abundant ion is C3H9N+, with 59 m/z of mass. Above this energy the most abundant ion is C3H8N+, with 58 m/z of mass (see Fig.1 and Tab.1).
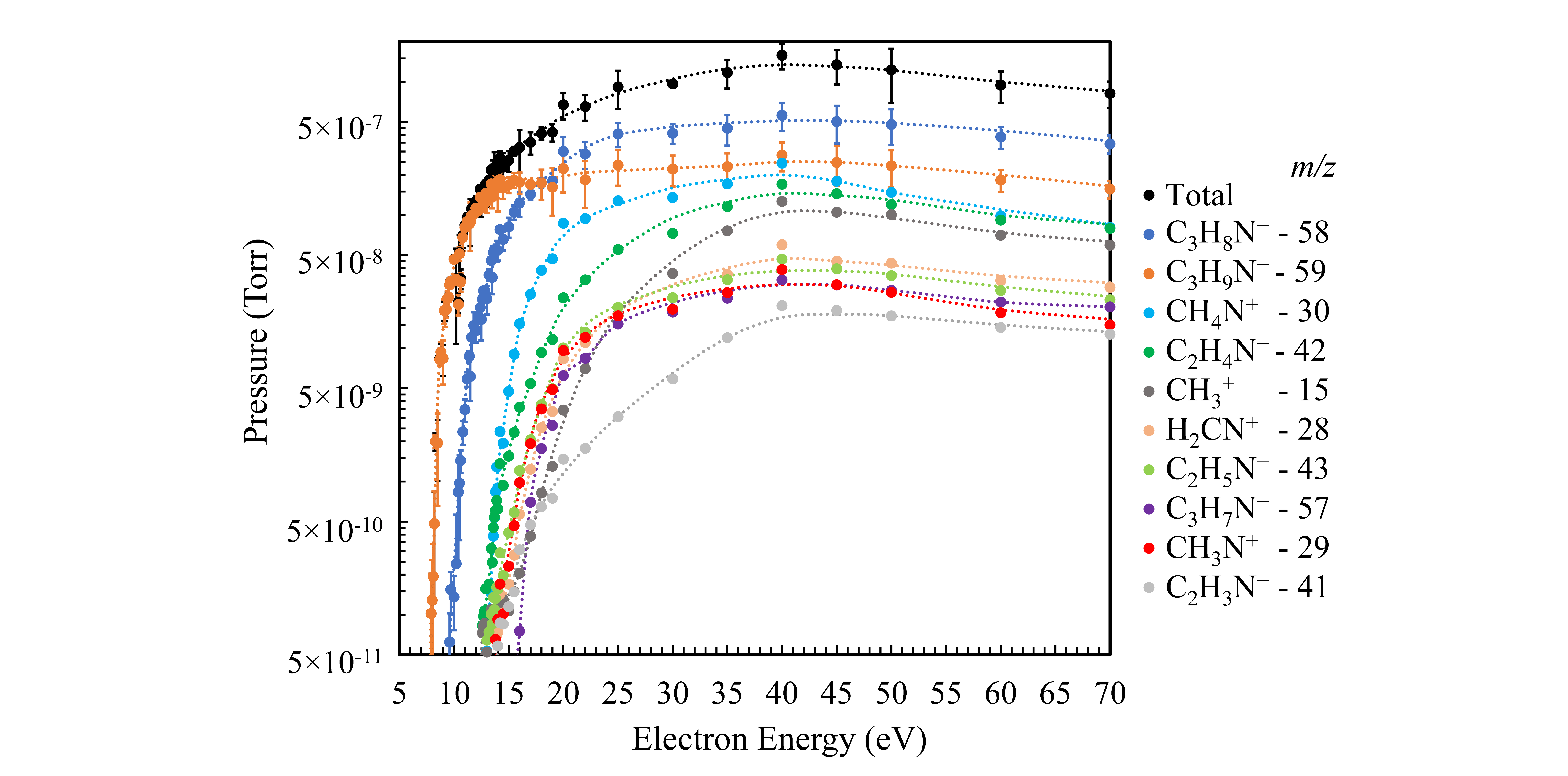
Figure 1: Partial pressure of the different ion species produced by electron impact in TMA [(CH3)3N] together with total ion pressure (as measured with the RGA), as a function of electron impact energy (up to 70 eV). Error bars are only represented for the total pressure and the two most abundant ions, as representing all error bars could threat the clarity of the figure. Dashed lines are guides to the eye.
Table 1 – Ionization energy of TMA and appearance energies of the ten most abundant ions produced in TMA by electron impact (in eV).
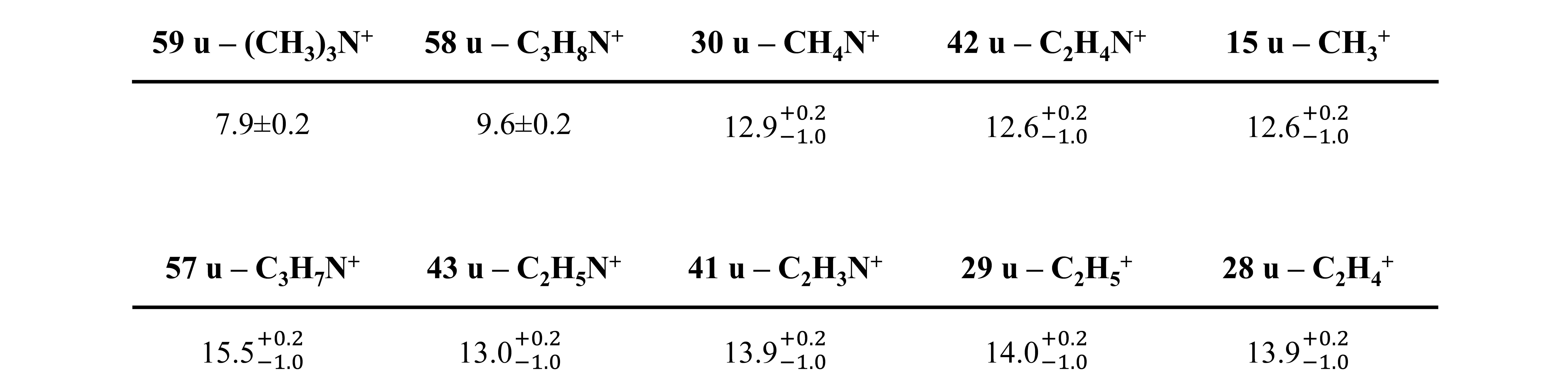
We believe that the appearance energies obtained with this method, using the Hiden quadrupole mass spectrometer model HAL 200 RC is a good estimate, even for the less abundant TMA ions analyzed (in the case of TMA m/z 30, 42, 15, 57, 43, 41, 29 and 28) that have a larger associated uncertainty due to the lower RGA sensitivity when working at higher pressures.
[1] D.R. Nygren, J. Phys.: Conf. Ser. 309 (2011) 012006.
 Project summary by: A.M.F. Trindade, Laboratório de Instrumentação e Física Experimental de Partículas, Departamento de Física, Universidade de Coimbra, Rua Larga, 3004-516 – Coimbra, Portugal
Project summary by: A.M.F. Trindade, Laboratório de Instrumentação e Física Experimental de Partículas, Departamento de Física, Universidade de Coimbra, Rua Larga, 3004-516 – Coimbra, Portugal
Paper Reference: A.M.F. Trindade et al., “Determination of ionizing threshold of trimethylamine ions with a high-resolution mass spectrometer” J. Instrum. 17 (2022) P02031 DOI: 10.1088/1748-0221/17/02/P02031
Hiden Product: HAL 200 RC
To find out more about these products visit the HAL 200 RC product page or if you would like to contact us directly please Send us a Message.

