The WGS reaction (CO + H2O → CO2 + H2) is an essential processing step in the industrial production of synthesis gas—one used both to purify and to adjust the H2 composition in important downstream processes such as the Fischer–Tropsch synthesis of hydrocarbons (CO:H2 = n:(2n + 1)), methanol synthesis (CO:H2 = 1:2), and the Haber–Bosch process for the production of ammonia. Thus, it is essential to develop effective catalysts with good catalytic properties for the WGS reaction. Metals such as Au, Cu or Pt on reducible oxides such as CeO2 and titania based nanocatalysts are very promising new candidates for high activity, lower temperature WGS systems. These metal/oxide catalysts exhibit a substantially higher activity than the traditional Cu/ZnO WGS catalysts. The design and optimization of the ceria and titania based WGS catalysts is hindered by controversy about basic questions regarding the nature of the active species/sites and the reaction mechanism. A central challenge is the difficulty of characterizing the active state of the catalyst, the reaction mechanism, and the associated reaction intermediates, at the elevated pressure and temperatures for WGS operation.
We recently studied the Pt-CeO2 nanocatalysts (Nature Commun. 12, 914 (2021)) with promising WGS activity. The reactants and products were analyzed by using a mass spectrometer (Hiden QGA). The structural features of the catalysts were characterized by using a combination of multimodal characterization and we demonstrated how the specific features of the active metal-support interfacial bonding—perhaps most importantly the temporal dynamic changes occurring therein—serve to enable high activity. The dynamic characteristics of a Pt/CeO2 system at the atomic level remains complex for the WGS reaction and specifically reveal the synergistic effects of metal-support bonding at the perimeter region. For instance, we find that the perimeter Pt0−O vacancy−Ce3+ sites are formed in the active structure, transformed at working temperatures and their appearance regulates the adsorbate behaviors. We find that the dynamic nature of this site is a key mechanistic step for the WGS reaction.
Dynamics and heterogeneity of atoms in the active Pt species were probed with a multitude of techniques. In situ TEM measurements observed the atoms within active Pt nanoparticles supported on Ceria initially under CO and then under WGS. Under CO the Pt structure is barely discernable, likely due to rapid CO induced mobility accompanied by the weakening of the Pt–Pt bonds from the strong repulsive interactions occurring between adsorbed CO molecules and because of the elevated temperature. Pt atoms are dynamic under CO at 200 °C, notably for those atoms deep in the nanocluster core and at/close to the interface with oxide. In contrast, under the WGS, almost all the outer Pt atoms become more stabilized/localized except for the ones located at the perimeter sites of the cluster. These observations suggest complexity within the nanocluster dynamically and heterogeneously change under in situ conditions. A multitude of complimentary probes were also applied to elucidate the changes to the Pt and Ceria actives sites under reaction conditions. The complexity involves the structural evolution of the catalysts, heterogeneity and dynamic behavior of atoms located at different places of the nanostructure. The structural features and details of how the dynamic changes are connected to reactivity will help the scientists understand the working mechanism of this particular catalyst and potentially design ones with better activity at lower cost. The same techniques can also be applied to studies of other catalysts.
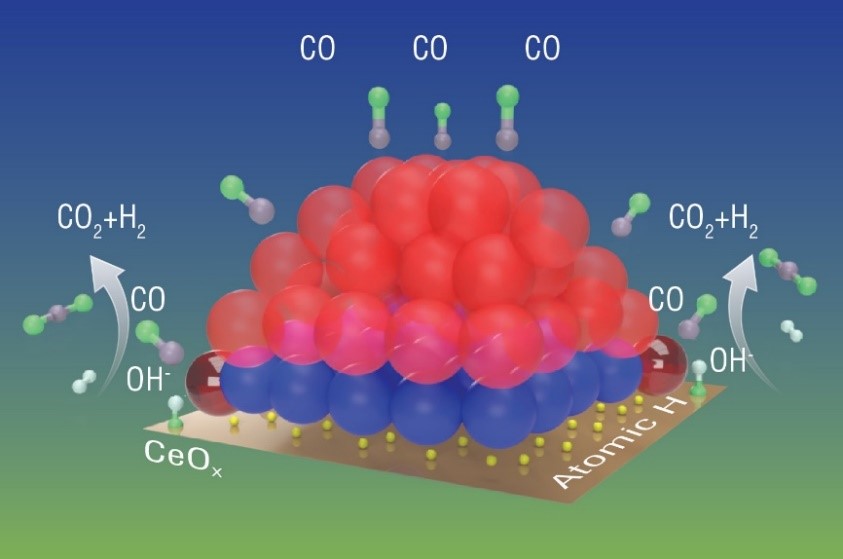
The schematic of a working Pt/CeO2 catalyst under the WGS reaction. The red and blue spheres represent Pt atoms that are metallic (zero state) and oxidized (2+), respectively. Under the reaction condition, only some platinum atoms around the periphery of the nanoparticle (shiny dark red) get activated to take part in the reaction. These activated platinum atoms transfer oxygen from OH groups (originally from water molecules) to carbon monoxide (CO), transforming it to CO2, leaving the H to combine with atomic hydrogen to form H2.
Project summary by: Yuanyuan Li, Department of Materials Science and Chemical Engineering, Stony Brook University, Stony Brook, NY 11794, USA.
Paper Reference: “Dynamic structure of active sites in ceria-supported Pt catalysts for the water gas shift reaction” Nature Communications (2021) 12, 914 https://doi.org/10.1038/s41467-021-21132-4
Hiden Product: QGA
Project Summary Reference: AP-QGA-202113
To find out more about these products visit the QGA product page or if you would like to contact us directly please Send us a Message.

