 We focused on steam reforming of biomass tar, which is an alternative to fossil fuels and includes toluene-like compounds in its structure. Generally, for Ni supported on metal oxide (Ni/metal oxide), aromatic hydrocarbons tend to form carbon covered active sites of catalyst and this deactivates the catalyst. Nevertheless, it was revealed that Ni/La0.7Sr0.3AlO3-δ has high activity and low carbon deposition during toluene steam reforming. In this reaction, lattice oxygen plays an important role for oxidizing surface carbon and decomposing reactant toluene. Therefore, we needed to confirm the relationship between lattice oxygen release rates from the metal oxide and reaction rates, amounts of deposited carbon by detecting the emission behavior of lattice oxygen in/on the perovskite oxide.
We focused on steam reforming of biomass tar, which is an alternative to fossil fuels and includes toluene-like compounds in its structure. Generally, for Ni supported on metal oxide (Ni/metal oxide), aromatic hydrocarbons tend to form carbon covered active sites of catalyst and this deactivates the catalyst. Nevertheless, it was revealed that Ni/La0.7Sr0.3AlO3-δ has high activity and low carbon deposition during toluene steam reforming. In this reaction, lattice oxygen plays an important role for oxidizing surface carbon and decomposing reactant toluene. Therefore, we needed to confirm the relationship between lattice oxygen release rates from the metal oxide and reaction rates, amounts of deposited carbon by detecting the emission behavior of lattice oxygen in/on the perovskite oxide.
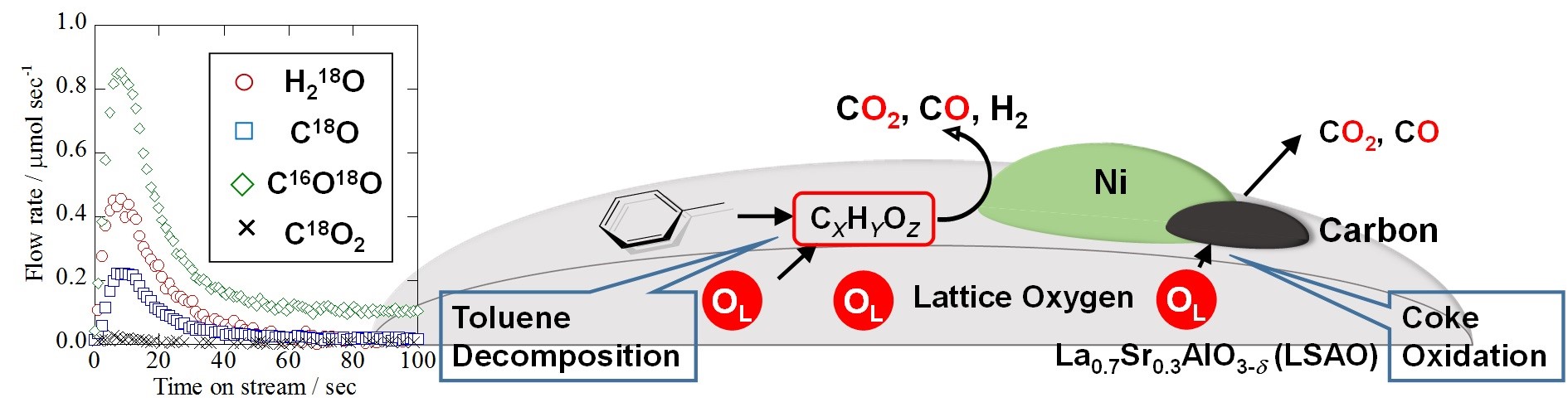
Fig. 1 Lattice oxygen release monitored by the Quadrupole mass spectrometer and reaction mechanism
To elucidate the emission behavior of lattice oxygen, H218O SSITKA (Steady State Isotopic Transient Kinetic Analysis) was adopted using Hiden HPR-20 system. This isotopic transient test was conducted according to the following procedure: the first reaction gas including H218O: composite gas comprising C7H8: H216O: H218O: Ar: He = 1.5%: 6.3%: 14.7%: 5%: 72.5% (total: 200 mL/min) was introduced into a tubular reactor for 30 min to replace the lattice oxygen in/on the perovskite oxide by 18O. After the first reaction, surface adsorbents were purged by inert gas for 120 min. Subsequently, the second reaction gas not including the isotope: composite gas comprising C7H8: H216O: Ar: He = 1.5%: 21%: 5%: 72.5% (total: 200mL/min) was flowed into the reactor; simultaneously release rates of lattice oxygen were calculated by isotopic generated gas, i.e. C18O, C16O18O, C18O2 measured with a Quadrupole Mass Spectrometer (HPR-20 QIC; Hiden Analytical Ltd). This experimental method enables investigation of lattice oxygen release and compares it with several catalysts having different structures.
Release of lattice oxygen was observed only for Ni metal supported on metal oxides like La0.7Sr0.3AlO3-δ, which suggests that the existence of Ni is significant for lattice oxygen release. Thereby, the calcination temperature of the Ni nitrate (the precursor of Ni) was changed to control Ni particle size on each Ni/La0.7Sr0.3AlO3-δ catalyst. Consequently, each catalyst had different Ni metallic surface area and perimeter between Ni and metal oxide. As the calcination temperature of supported Ni decreased from 1373 K to 1073 K, the release rate of lattice oxygen increased from 22.8 to 64.0 mol sec-1 g-cat-1. Concurrently, toluene conversion and hydrogen yield drastically increased from 4.7% to 49.9% and from 4.1% to 41.9%, respectively. On the other hand, the carbon deposition declined significantly from 111.1 to 29.9 mg g-cat-1. According to these results, lattice oxygen enhances the catalytic activity and stability, oxidizing surface carbon and toluene.
Additionally, we evaluated the lattice oxygen release rate and catalytic activity per unit of Ni surface and perimeter respectively. There was better correlation between lattice oxygen release rate and catalytic activity per unit of perimeter i.e. mmol m-1 sec-1 for C1 gas yield. Therefore, in conclusion, we hypothesize release of lattice oxygen occurs mainly at at the interface between Ni particles and the metal oxide, La0.7Sr0.3AlO3-δ.
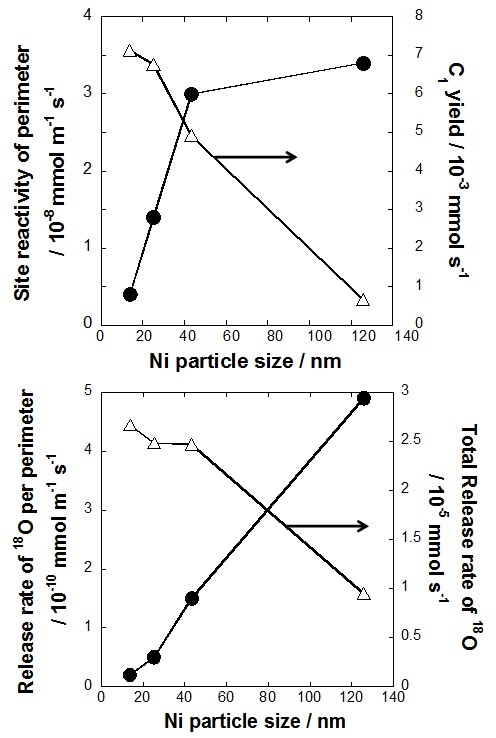
Fig. 2 Effect of Ni particle size on the redox properties and reactivity of the catalyst.
Project summary by:
Yasushi Sekine
Department of Applied Chemistry
3-4-1, Okubo, Shinjuku
Tokyo, 169-8555
Japan
Paper Reference:
Kento Takise, Masaya Imori, Daiki Mukai, et al. (2015) “Effect of catalyst structure on steam reforming of toluene over Ni/La0.7Sr0.3AlO3−δ catalyst” Applied Catalysis A: General 489, 155-161

