Increasing emissions of nitrogen oxides associated with global industrialization necessitates the development of more sustainable NOx-removal systems. This research focused on synthesizing and testing catalytic materials, derived from biomass waste and Earth-abundant metals. These materials were sufficiently reactive to convert NOx into nitrogen, employing hydrogen as the reductant. A catalytic activity testing rig was developed for this purpose and was able to continuously monitor the concentration of NOx in the effluent, alongside the formation of by-products including ammonia and nitrous oxide. The catalysts in this study were prepared from palm kernel shell (PKS) activated carbon impregnated with copper and iron oxides at various compositions. Synthetic NOx gas was passed through the catalyst bed at a controlled temperature. A mass spectrometer MS (Hiden HPR-20, UK) provided on-line monitoring of the concentration of products using m/z ratios of 16, 17, 18, 28, 30, 44, 46 for oxygen, ammonia, water, nitrogen, nitric oxide, nitrous oxide and nitrogen dioxide respectively. Due to the compounds of interest presenting overlapping fragmentation patterns, calibration of the MS using the individual components was performed allowing deconvolution of the data. The catalytic activity of the PKS-supported copper-iron catalyst is shown in Figure 1. It can be seen that total conversion of nitric oxide was achieved beyond 150 °C, however this coincides with the formation of ammonia and nitrous oxide. The catalyst design can be improved to inhibit the formation of these by-products. These findings open the door for opportunities to develop a catalyst system that utilizes biomass waste and a renewable reductant such as green or blue hydrogen, which is cost-effective and attractive industrially [1]. 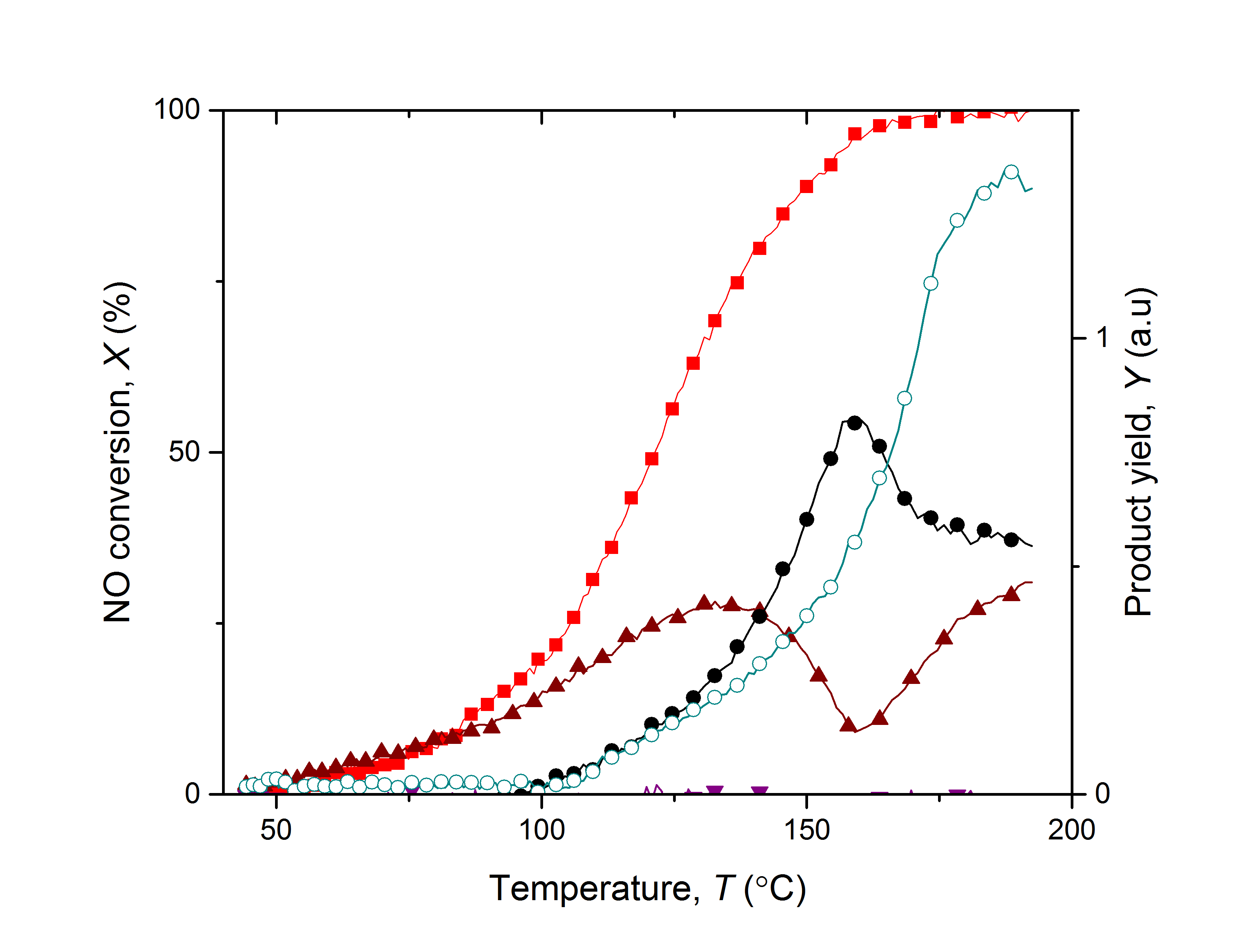
Figure 1. Temperature-programmed reaction profiles for PKSCuFe. 250 sccm total flow of 330 ppm NO + 8000 ppm H2 at atmospheric pressure and increasing temperature from 40 to 200 °C by 2 °C/min over 1.0 g catalyst. (■Converted nitric oxide, ▲Nitrous oxide, ●Nitrogen, ○Ammonia, ▼Nitrogen dioxide) [1]
 Project summary by: Dr Ibrahim Yakub, Department of Chemical Engineering & Energy Sustainability, Faculty of Engineering, Universiti Malaysia Sarawak (UNIMAS) 94300 Kota Samarahan Sarawak, Malaysia.
Project summary by: Dr Ibrahim Yakub, Department of Chemical Engineering & Energy Sustainability, Faculty of Engineering, Universiti Malaysia Sarawak (UNIMAS) 94300 Kota Samarahan Sarawak, Malaysia.
Paper Reference: [1] Yakub I, Barawi MH, and McGregor J, “Assessment of copper-iron catalyst supported on activated carbon for low-temperature nitric oxide reduction by hydrogen,” IOP Conf. Ser. Earth Environ. Sci., vol. 765, no. 1, p. 012093, 2021.
Hiden Product: HPR-20
Reference: AP-HPR-20-202181
To find out more about these products visit the HPR-20 R&D product page or if you would like to contact us directly please Send us a Message.

