 Diesel engines are becoming ever more popular for automotive applications principally due to their higher fuel efficiency. However, the increasingly stringent emission legislation has created a need to develop improved after-treatment systems. HC-SCR technology offers the potential to reduce NOx emissions using un-burnt hydrocarbons in a compression ignition diesel injection engine exhaust stream, negating the need for an external reductant such as ammonia. It has the potential to reduce the overall capital cost of a vehicle as it does not require the additional infrastructure associated with the current state-of-the-art (NH3-SCR) system. Furthermore it allows for the simultaneous removal of both NOx and un-burnt hydrocarbons from the exhaust owing to a more intensified and robust emission control system. However for this technology to become a commercially attractive competitor to the NH3-SCR system, it must demonstrate activity at low temperatures and be able to operate across a wide temperature range.
Diesel engines are becoming ever more popular for automotive applications principally due to their higher fuel efficiency. However, the increasingly stringent emission legislation has created a need to develop improved after-treatment systems. HC-SCR technology offers the potential to reduce NOx emissions using un-burnt hydrocarbons in a compression ignition diesel injection engine exhaust stream, negating the need for an external reductant such as ammonia. It has the potential to reduce the overall capital cost of a vehicle as it does not require the additional infrastructure associated with the current state-of-the-art (NH3-SCR) system. Furthermore it allows for the simultaneous removal of both NOx and un-burnt hydrocarbons from the exhaust owing to a more intensified and robust emission control system. However for this technology to become a commercially attractive competitor to the NH3-SCR system, it must demonstrate activity at low temperatures and be able to operate across a wide temperature range.
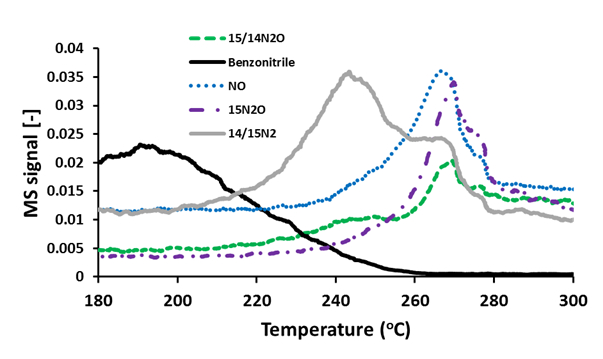
Figure 1: The evolution / consumption of various reactants and products as a function of temperature for the reaction between 14N-benzonitrile and isotopically labelled 15NO over 0.5wt%Au.0.5wt%Pd / TiO2. The corresponding m/z values measured were as follows: 14/15N2 (m/z:29), 14/15N2O (m/z:45),14NO (m/z:30), 15/15N2O (m/z:46), 14N-benzonitrile (m/z:103).
In our study we utilise the ability of gold and palladium bimetallic catalysts to selectively oxidise hydrocarbons to enhance the hydrocarbon selective catalytic reduction of NOx, a reaction in which the interaction of partial oxidation intermediates is considered important. We report a synergetic effect associated with alloying Au with Pd, producing a catalyst that is more active for NOx conversion than the corresponding mono-metallic materials. Furthermore we demonstrate that the interaction of partially oxygenated species with NO over these catalysts yields gas phase benzonitrile. Using our Hiden HPR-20 mass spectrometer to conduct steady state isotopic analysis we exclusively display that the direct interaction of this nitrile with NO leads to the formation of N2and N2O (see Figure 1). Analysis of the results suggests that there may be two competing parallel reaction pathways for the consumption of these –CN species, both of which are critical in dictating the N2 selectivity of a HC-SCR catalyst. The nitrile may be readily reduced by NO to produce N2, or it may be further oxidised leading to formation of N2O. It has been demonstrated that a catalyst with better combustion activity is more selective to N2O production. Due to the high levels of N2O production, along with the various other gas phase species present, this gold and palladium alloyed catalyst is not suitable for commercial application. However it is possible to utilise the findings of this study to develop a catalyst that allows functional control over the rates of both of these parallel reactions, yielding a more selective low temperature HC-SCR catalyst.
Project Summary by:
Conor Hamill
CenTACat
School of Chemistry and Chemical Engineering
Queen’s University Belfast
Belfast, BT9 5AG
UK
Reference:
Conor Hamill et al. (2014) “Evaluation and mechanistic investigation of a AuPd alloy catalyst for the hydrocarbon selective catalytic reduction (HC-SCR) of NOx” Applied Catalysis B: Environmental 147, 864-870

