Mechanochemistry is a discipline of chemistry that examines chemical reactions that take place when mechanical energy is applied. Since the 1860s, mechanochemical processes have had a growing influence on a variety of technological disciplines, including powder processing. These approaches have found use in the fabrication of fine and homogeneous powder mixtures as well as the synthesis of sophisticated materials.
High-energy mills, such as planetary mills, roto-vibrational mills, vibration mills, attritor mills, pin mills, and rolling mills, are commonly used in mechanochemical processes.
The energy transferred as a result of collisions between powder particles and chamber in a milling process has never been considered as important to the milling process’ outcome. This is mostly due to the expected low relevance that the energy transferred through these collisions has on the overall mechanochemical process when compared to the energy transferred during the impacts of the milling media with the chamber walls.
The goal of this study was to see how the energy transferred during powder-to-wall collisions in a milling process affects the milling outcome. As a model process, the synthesis of Mg(BH4)2 from MgB2 in a compressed hydrogen atmosphere was chosen. MgB2 was milled under a broad set of different milling conditions (i.e. by varying the two parameters milling times and rotation regimes) and the obtained products thoroughly characterized.
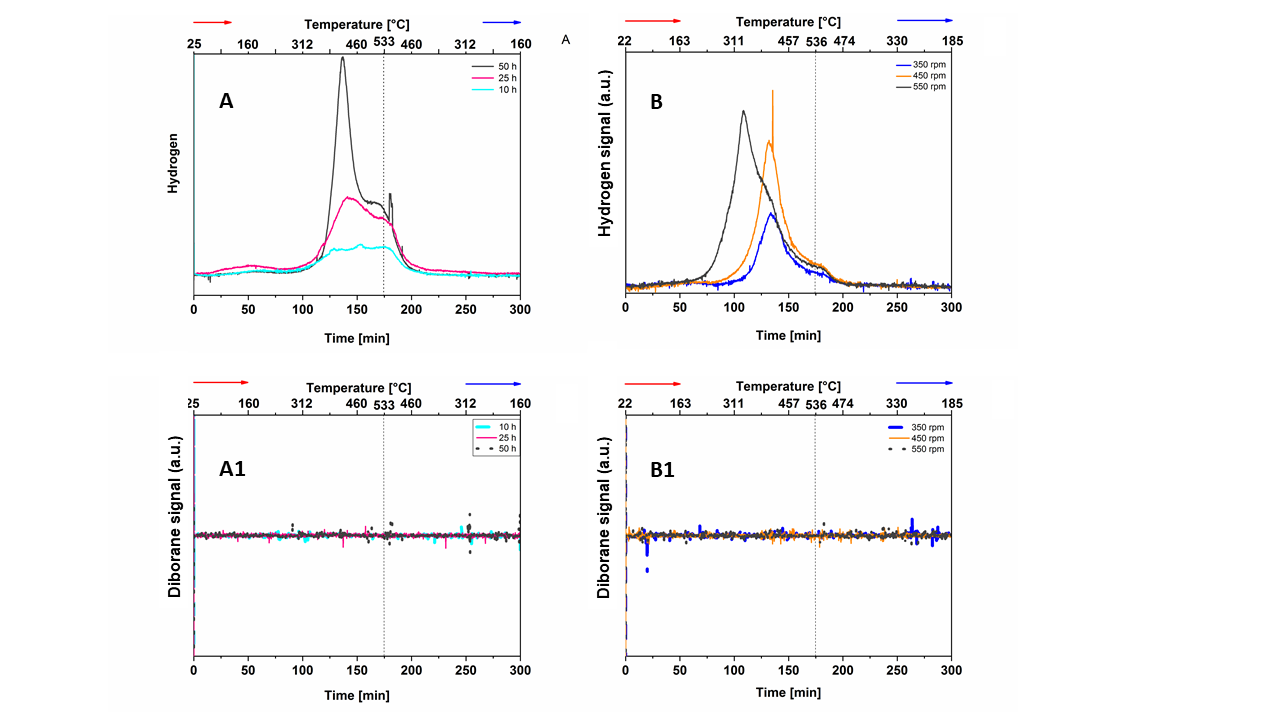
A Hiden Analytical HPR-20 Mass-Spectrometer was used to examine the composition of the gaseous phase produced during the desorption reaction of the tested samples. The MS signals for H2 (A and B) and B2H6 (A1 and B1) measured for mechanically hydrogenated samples under various conditions are shown in the above-reported figure.
A series of Solid-State Nuclear Magnetic Resonance measurements, powder X-ray diffraction analyses, and numerical simulations have revealed that a fraction of the original MgB2 is transformed into Mg(BH4)2, α- or β-rhombohedral boron and Mg/MgH2 as a result of the mechanochemical treatment. To the best of the author’s knowledge, this is the first time an attempt was made to estimate the collision energy for each particle using kinematic models available in the literature. The energy transferred during a single powder-to-wall impact is 5-6 orders of magnitude smaller than the typical energy transferred during a similar milling process carried out in the presence of milling media, according to the results of this calculation for the applied measurement conditions and powder features.
By proving the partial formation of Mg(BH4)2, the results of this investigation indicate that the energy transferred to the powder bed by the powder particles colliding with the chamber wall during milling is not negligible, in particular when the milling process is protracted for a long period.
Project summary by: Claudio Pistidda Institute of Hydrogen Technology, Materials Design, Helmholtz-Zentrum hereon GmbH, Max-Planck Strasse 1, Geesthacht D-21502, Germany
Paper Reference: “Hydrogenation via a low energy mechanochemical approach: the MgB2 case” J. Phys. Energy (2021) 3, 4, 044001
Hiden Product: HPR-20
Reference: AP-HPR-20-202216
To find out more about these products visit the HPR-20 product page or if you would like to contact us directly please Send us a Message.

