The ability of mixed ionic and electronic conducting (MIEC) perovskite oxides (ABO3) to support both electronic and ionic conductivity, as well as their favourable catalytic properties and chemical and redox stability, make them promising electrode materials. MIEC perovskite oxides have received substantial attention in multiple applications in energy conversion and storage devices, such as solid oxide fuel/electrolysis cells (SOFC/SOEC), oxygen transport membranes (OTMs), electrochemical sensors, and electrocatalysts for water splitting. In applications such as these, the rate of oxygen exchange across the gas – MIEC solid interface under various conditions often determines the overall performance of the device. The surface exchange mechanisms and kinetics have therefore been the focus of many studies. However, the majority of research has been carried out under dry oxygen environments, while practical operating conditions can be significantly more complicated.
Our research has focussed on the oxygen transport properties of MIEC perovskite oxides under humid environments. Water vapour is a fundamental oxygen bearing molecule present in the input gas stream in solid-state electrochemical systems, which acts as an electrolysis source for hydrogen production. Taking (La0.8Sr0.2)0.95Cr0.5Fe0.5O3-δ (LSCrF8255) as a model MIEC perovskite oxide, the oxygen transport properties were probed through isotope exchange depth profiling (IEDP) coupled with secondary ion mass spectrometry (SIMS) [1, 2]. The exchanges were carried out in water vapour (pO2 < 1 mbar, pH218O = 30 mbar), dry oxygen (p18O2 = 200 mbar, pH2O = 0 mbar) and wet oxygen (pO2 = 200 mbar, pH218O = 30 mbar) atmospheres. In-situ residual gas analysis (RGA, HALO51, Hiden Analytical Ltd, UK) was also carried out during the exchange anneal to investigate the change in gas phase composition and to further deduce the water surface exchange mechanisms.
Figure 1 presents the Arrhenius plots of the surface exchange coefficients, k, obtained in the three conditions.
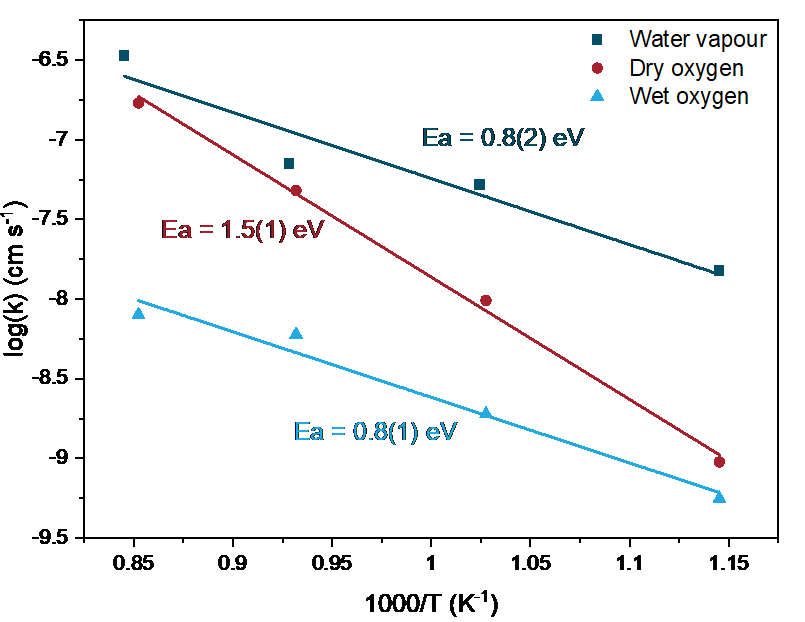 Figure 1. Surface exchange coefficients of LSCrF8255 samples isotopically exchanged in water vapour, dry oxygen, and wet oxygen atmospheres as a function of inverse temperature. Lines are least squares fits to Arrhenius behaviour. Reprinted with permission from Z.Sha et al, Chem. Mater. 2021, doi:10.1021/acs.chemmater.1c02909, Copyright 2021 American Chemical Society.
Figure 1. Surface exchange coefficients of LSCrF8255 samples isotopically exchanged in water vapour, dry oxygen, and wet oxygen atmospheres as a function of inverse temperature. Lines are least squares fits to Arrhenius behaviour. Reprinted with permission from Z.Sha et al, Chem. Mater. 2021, doi:10.1021/acs.chemmater.1c02909, Copyright 2021 American Chemical Society.
In Figure 1, an increase in k of up to 2 orders of magnitude was observed on the samples annealed in water vapour, and a decrease in activation energy of 0.7 eV was observed for water surface exchange compared to oxygen surface exchange. The significant enhancement in k is due to the greater oxygen vacancy concentration formed on the sample surface in water vapour, as well as the differing surface exchange mechanisms of water and oxygen molecules studied through in-situ RGA. A potential mechanism for water surface exchange on MIEC electrodes is described in Equations 1 and 2 and illustrated in Figure 2, in which the adsorption and dissociation of H218O in the gas phase occurs on a surface oxygen vacancy site to form hydroxyl groups, and further incorporation of the 18O ions occurs via desorption of H216O molecules [2].
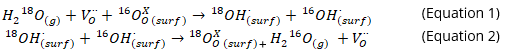
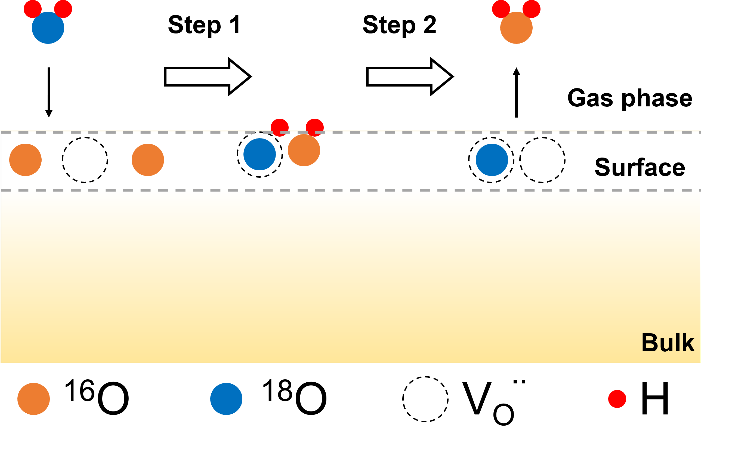
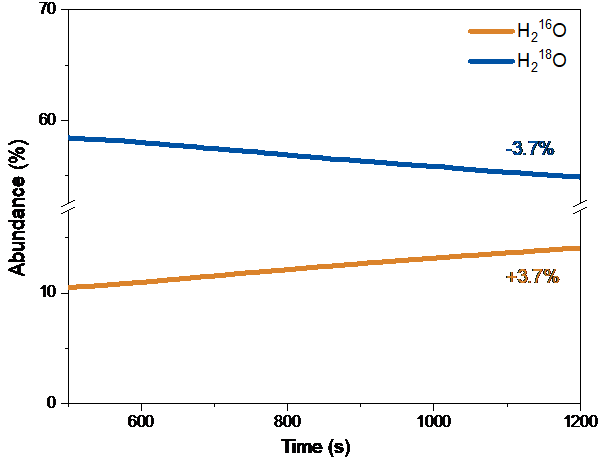
Figure 2: Schematic of the surface exchange process and typical RGA data for the labelled water species. Reprinted with permission from Z.Sha et al, Chem. Mater. 2021, doi:10.1021/acs.chemmater.1c02909, Copyright 2021 American Chemical Society.
The non-redox oxygen exchange, in contrast to the oxygen reduction reactions (ORR), and the formation of the hydrogen bonds, could lead to the enhanced surface exchange properties. In addition, the lowest k values were found on the samples exchanged in the wet oxygen atmosphere. This is due to the competition between water and oxygen molecules for the surface oxygen vacancy sites, as well as the molecular scrambling behaviour between H218O and 16O2 forming 16,18O2 probed through in-situ RGA [1]:
![]()
Our studies demonstrate that water-induced oxygen transport kinetics and mechanisms can be significantly discrete from those of molecular oxygen, leading to a general design guideline for the performance of MIEC electrodes. Using both SIMS and in-situ gas phase analysis provided the detail required to explain the exchange phenomena in this complex environment.
References:
- Sha, Z., Cali, E., Kerherve, G. and Skinner, S.J., 2020. Oxygen diffusion behaviour of A-site deficient (La0.8Sr0.2)0.95Cr0.5Fe0.5O3-δ perovskites in humid conditions. Journal of Materials Chemistry A, 8(40), pp.21273-21288.
- Sha, Z., Cali, E., Shen, Z., Ware, E., Kerherve, G. and Skinner, S.J., 2021. Significantly Enhanced Oxygen Transport Properties in Mixed Conducting Perovskite Oxides under Humid Reducing Environments. Chemistry of Materials.
 Project summary by: Zijie Sha and Stephen J. Skinner, Department of Materials, Imperial College London, Exhibition Road, London, SW7 2AZ, UK
Project summary by: Zijie Sha and Stephen J. Skinner, Department of Materials, Imperial College London, Exhibition Road, London, SW7 2AZ, UK
Paper Reference: “Significantly Enhanced Oxygen Transport Properties in Mixed Conducting Perovskite Oxides under Humid Reducing Environments” Chem. Mater. (2021) 33, 21, 8469-8476
DOI: 10.1021/acs.chemmater.1c02909
Hiden Product: RGA
Reference: AP-RGA-202108
To find out more about these products visit the RGA product page or if you would like to contact us directly please Send us a Message.

