 This research project concerns the abatement of H2S from a biogas stream by partial and selective oxidation to sulphur and water.
This research project concerns the abatement of H2S from a biogas stream by partial and selective oxidation to sulphur and water.
Hydrogen sulfide (H2S) is one of the most toxic compounds usually present in fuels, oil and gas refinery processes. In particular, it’s also present in little amount in biogas which is a renewable energy carrier obtained from the anaerobic digestion of organic substrates. The main compounds are CH4, CO2 but there are also sulfur compounds present.
Next to the traditional catalytic oxidation processes such as the Claus process used for the abatement of H2S, an interesting one-step solution for the clean-up of biogas from H2S could be, for small plants, selective catalytic H2S oxidation to sulfur at low temperatures. The partial H2S oxidation reaction is carried out in presence of vanadium-based catalysts that were identified, from a previous screening of catalysts, to be very active and selective to sulphur.
The catalytic tests were carried out in a fixed bed flow reactor, inserted in an electrical furnace equipped with a PID electronic temperature controller. A thermocouple is inserted in a steel sheath of inner diameter of 6 mm concentric to the reactor. The catalytic tests were performed at atmospheric pressure with a contact time of 20 ms, at temperatures between 150 and 250°C, by feeding 200 ppm of H2S, 100 ppm of O2 and N2 to balance. The scheme of the laboratory plant is shown in Figure 1 (Fig.1).
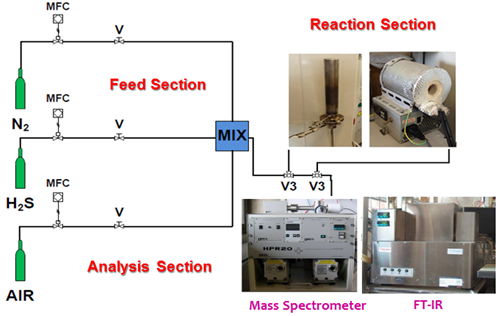
Fig. 1. Schematic diagram of the experimental apparatus
The exhaust stream (H2S, O2, H2O) was analyzed by a quadrupole mass spectrometer (Hiden HPR-20 QIC). It was equipped with a sulfur trap to prevent sulfur causing the occlusion of the capillary and damage to the fundamental parts of the analyzer. The concentration of SO2, which may be present at a very low concentration level in the stream leaving the reactor was monitored by an analyzer FT-IR Multi-gas in continuous, consisting of the spectrophotometer Nicolet Antaris IGS Thermo Electron, with a specific cell for gas.
In Figure 2 (Fig.2), the typical behavior of a catalytic test performed at 150 °C on the 20 wt% V2O5/CeO2 sample is shown, by plotting the concentration profiles of the reactants (H2S, O2) and the products (SO2, H2O) during the test. After 10 minutes, the feed stream was sent to the reactor after which it is possible to see a significant decrease of the H2S, O2 concentration with SO2 and water production.
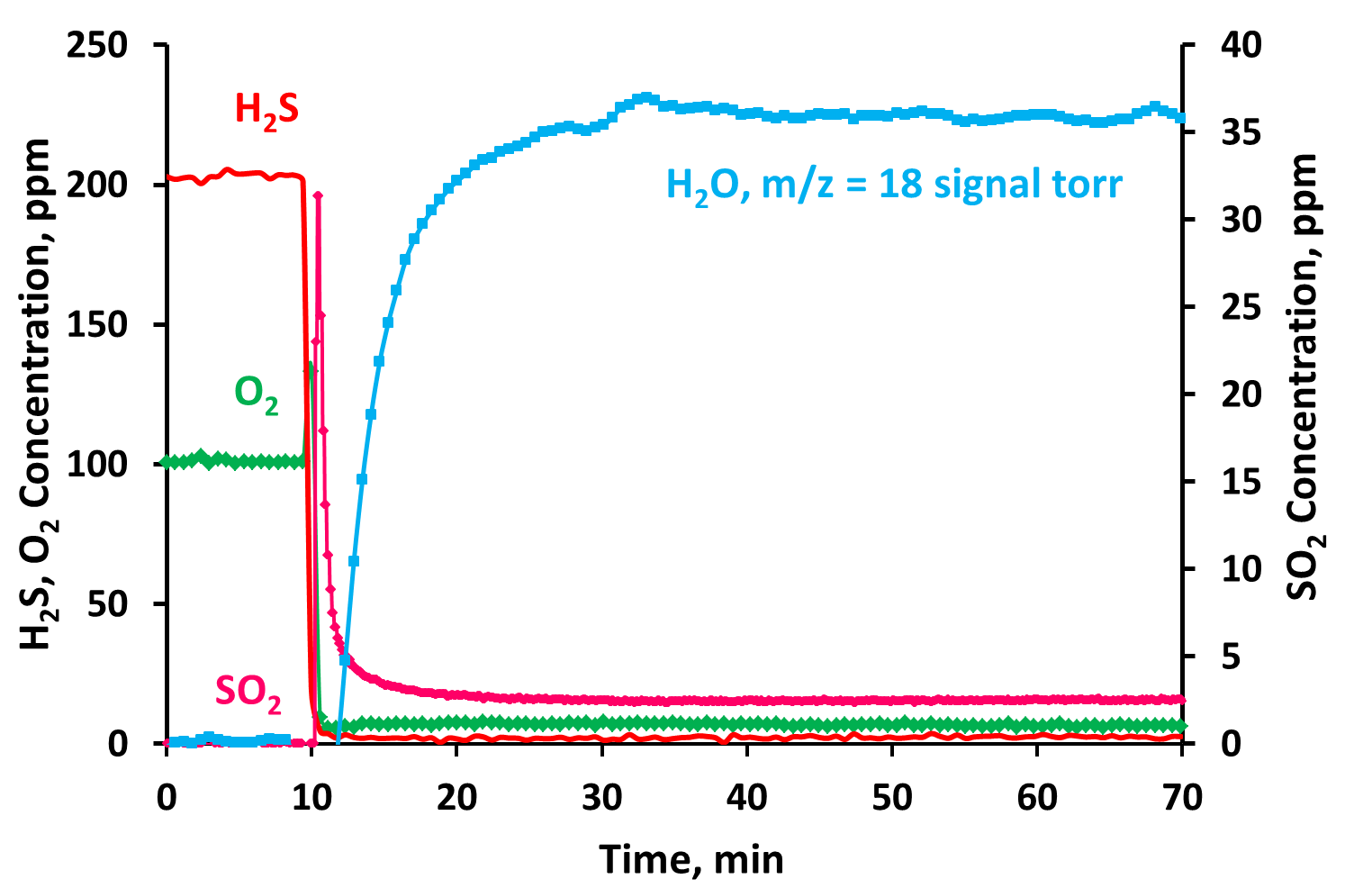
Fig.2. Catalytic activity test for the catalyst 20 wt% V2O5/CeO2 at 150°C.
The concentration values of H2S, O2 and SO2 reached a stationary value after 10 min on stream. The final H2S and O2 conversion values were 98% and 94% respectively, with a very low SO2 selectivity (1.5%).
The water formation, indicated by the behavior of the signal m/z =18, after the initial transient time of about 20 min, was stable during the overall test time.
The catalyst has shown a good catalytic activity without evident deactivation phenomena during the test time.
Project summary by:
Vincenzo Palma and Daniela Barba
Department of Industrial Engineering
University of Salerno
Via Giovanni Paolo II
132, Fisciano, SA, Italy
Paper Reference:
V. Palma & D. Barba (2014) “Low temperature catalytic oxidation of H2S over V2O5/CeO2 catalysts” International Journal of Hydrogen Energy 39 (36), 21524-21530

