As a successor of research based on metal borohydrides, higher boranes are being investigated. Ag2B12H12 and Ag2B10H10 are synthesized and found to possess very high ion conductivities, as well as being semiconducting. In the course of decades, research on borane materials has broadened to embrace many different properties other than hydrogen storage properties. This is an example of unravelling such useful properties, serving as a basis for further property studies of related materials.
Ag2B12H12 reduces to metallic silver, by exposure to an electron beam (TEM), and as such caught attention for further work. Silver boranes are also complexed with AgI in order to access high ion conductivities at room temperature. Synthesis is straight forward, and is achieved in water as a solvent, yielding the desired materials. It is crucial to ensure that residual water is fully removed, to perform reliable quantification of semi-conducting and ion conducting properties. For this purpose, mass spectrometry was employed, analyzing the release of any residual gases, providing assurance of completely dry and water free samples. The gas spectrum was also analyzed to determine the thermal stability of the silver boranes and understand their decomposition mechanism.
The Hiden Analytical HPR-20 quadrupole mass spectrometer was utilized and allowed for unambiguous determination of the water content, as well as ensuring that no undesired impurities are present. At the University of Aarhus, Department of Chemistry and iNANO center, daily investigations of e.g. borane materials are aided by analysis of residual gases released from materials studied as either classical hydrogen storage materials, or as demonstrated here, for other purposes. The information gained is valuable in evaluating synthesis methods, evaluating the purity of materials, and for studies of behavior upon thermal decomposition of materials.
Hydrogen evolution as the silver boranes decompose, is evident from the data presented in the DSC/MS figures. Both compounds decompose above 250oC, releasing an appreciable quantity of hydrogen. Heating to high temperatures for ion conductivity studies it is obvious that no decomposition or release of any gaseous species is observed. Thus, the information from the mass spectroscopy data assures that the samples are pure and that the derived properties are quantified accordingly.
Project Summary by:
Bo Richter and Torben R. Jensen
Department of Chemistry and Interdisciplinary Nanoscience Center (iNANO), Center for Materials Crystallography (CMC), Aarhus University, Denmark.
www.chem.au.dk / www.inano.au.dk



Paper Reference:
“Multifunctionality of silver closo-boranes” Nature Communications 8, Article number: 15136 (2017). doi:10.1038/ncomms15136
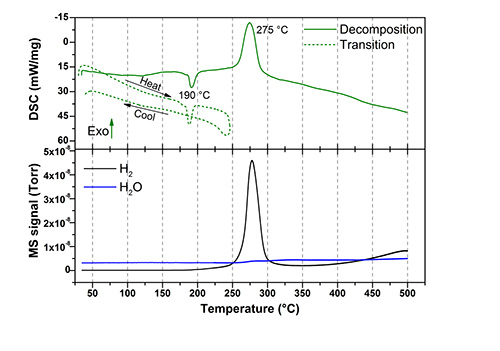
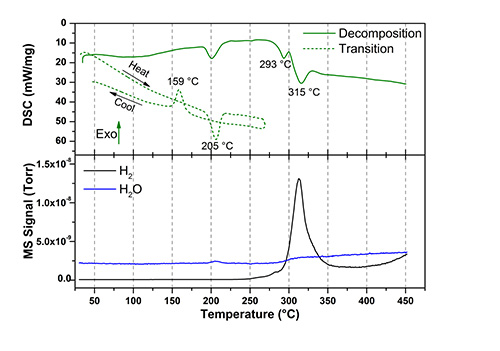
Differential scanning calorimetry (DSC) and mass spectroscopy (MS) data of Ag2B10H10 (top) and Ag2B12H12 (bottom). Two DSC measurements are shown for each material, one illustrating decomposition (solid line) and another showing the polymorphic phase transformation (dashed line). The MS trace is shown in the bottom part of the graphs.

