Acid catalysts have been the backbone of the chemical industry since the very first plants in which they were called to convert oil into precious products. With the progressive shift towards sustainable resources, often the old catalysts fail since biomass-based compounds feature different functional groups, each requiring a dedicated catalytic functionality to be effectively converted into the desired products. The development of new catalytic materials is therefore critical. In the work, we developed a series of zirconia catalysts modified with different loadings of silica showing mixed acid-based properties depending on the relative content of silica. We observed an unexpected surge in acidity at a certain Zr/Si ratio, specifically of the Brønsted type, mirrored by a shift in the catalytic activity. In detail, by using these acidic catalysts we were able to convert molecules such as HMF and furfural, which can be derived directly from biomass, into the corresponding ethers that are of high interest as biofuels. Since part of this process is catalyzed by the acidic functionalities of the catalysts, we used a probe reaction to assess such features, namely the 2-propanol dehydration to propene. The catalysts were impregnated with 2-propanol and then the temperature was raised under a continuous inert gas flow. The desorption of 2-propanol and the formation of propene along with acetone as possible side product, were followed with a Hiden HPR-20 mass spectrometer. The main mass signals of these compounds were recorded as function of time and then they were correlated to the temperature (Figure 1). As expected, the catalyst containing a certain silica amount, herein named as ZrSi30, and having the larger amount of acid sites—and, specifically, Brønsted ones–outperformed the bare zirconia by dehydrating the 2-propanol at a much lower temperature (220 °C vs 340 °C). This can be seen in the profile of the propene signal in the figure whose maximum appears sooner than that of the bare zirconia catalyst, meaning that the dehydration reaction was triggered at lower temperature. The relative simplicity of this kind of probe reaction, together with the ease in operating and versatility in setting up of the Hiden HPR-20 mass spectrometer, allowed us to quickly assess the acid character of these and many other materials for other applications.
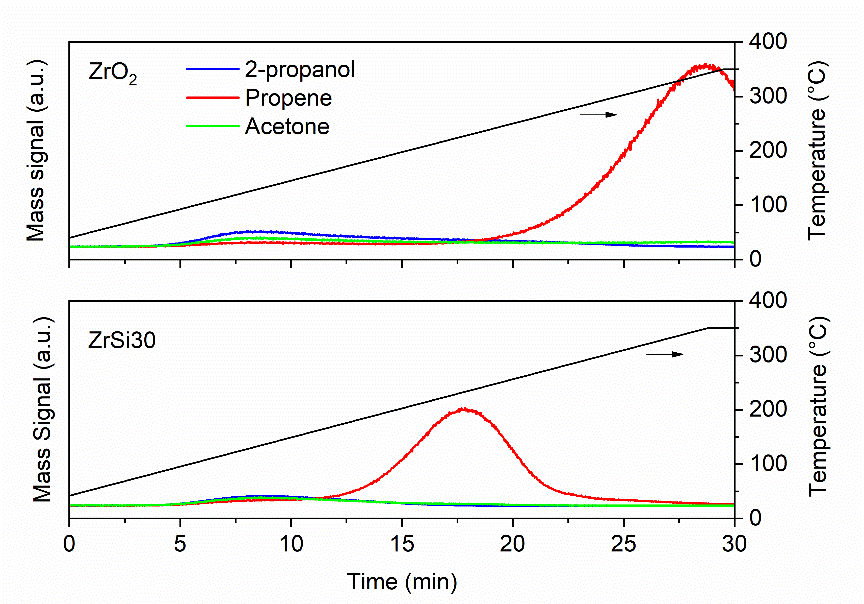
Figure 1. Comparison of the 2-propanol dehydration power of zirconia and zirconia-silica catalysts.
Project summary by: Filippo Bossola, CNR, Istituto di Scienze e Tecnologie Chimiche “Giulio Natta”, (SCITEC), via Golgi 19, 20133 Milano, Italy
Paper Reference: “On demand production of ethers or alcohols from furfural and HMF by selecting the composition of a Zr/Si catalyst” Catalysis Science & Technology, 2020 (10) 7502-7511 https://doi.org/10.1039/D0CY01427C
Hiden Product: HPR-20
Project Summary Reference: AP-HPR-20-202003
To find out more about these products visit the HPR-20 product pages or if you would like to contact us directly please Send us a Message.

