Ammonia borane (NH3BH3, AB hereafter) is a white crystalline inorganic solid with 19.6 wt. % mass content of hydrogen whose thermal decomposition releases up to 2 equivalents of hydrogen below 200ºC and therefore constitutes a promising hydrogen storage material. However, the application of AB is hindered by slow hydrogen release kinetics among other factors. Confinement of AB in scaffolding nanoporous materials has shown to improve its performance in terms of lower decomposition temperature, better kinetics and suppression of volatile impurities. However, hydrogen release mechanisms are still unclear [1].
In this work, hydrogen thermal desorption from AB, which has been incorporated into various mesoporous carriers, has been investigated by means of operando Raman – Mass Spectrometry methodology which consists in using a combination of real-time Raman spectroscopy measurements and simultaneous on-line analysis of the effluents by a Hiden HPR-20 quadrupole mass spectrometer [2]. This technique allows the study of the relationship between the structural/compositional changes in AB with hydrogen desorption properties in order to elucidate the mechanisms of decomposition, as well as, to clarify the benefits of dispersion and destabilization of AB by nanoconfinement.
The fixed-bed catalytic reactor employed in this work is a spectroscopic Raman cell that also permits the acquisition of high-quality spectra during the thermal decomposition of AB. This system is heated with an ad-hoc oven and two K-type thermocouples are introduced into the sample bed to monitor/control the reactor temperature and to provide an auxiliary temperature in the online mass spectrometer (Figure 1). Four scaffolds with different chemical composition and textural properties (pore diameter and volume) have been studied: i) Cobalt-substituted AlPO4-5 having intercrystalline mesoporosity, ii) a mesoporous gallium oxide, and two SBA-15 type materials denoted as iii) SBA-15-Linton and iv) SBA-15-Zhao. Hereafter these samples are named as AB-Co, AB-Ga, AB-L and AB-Z, respectively.
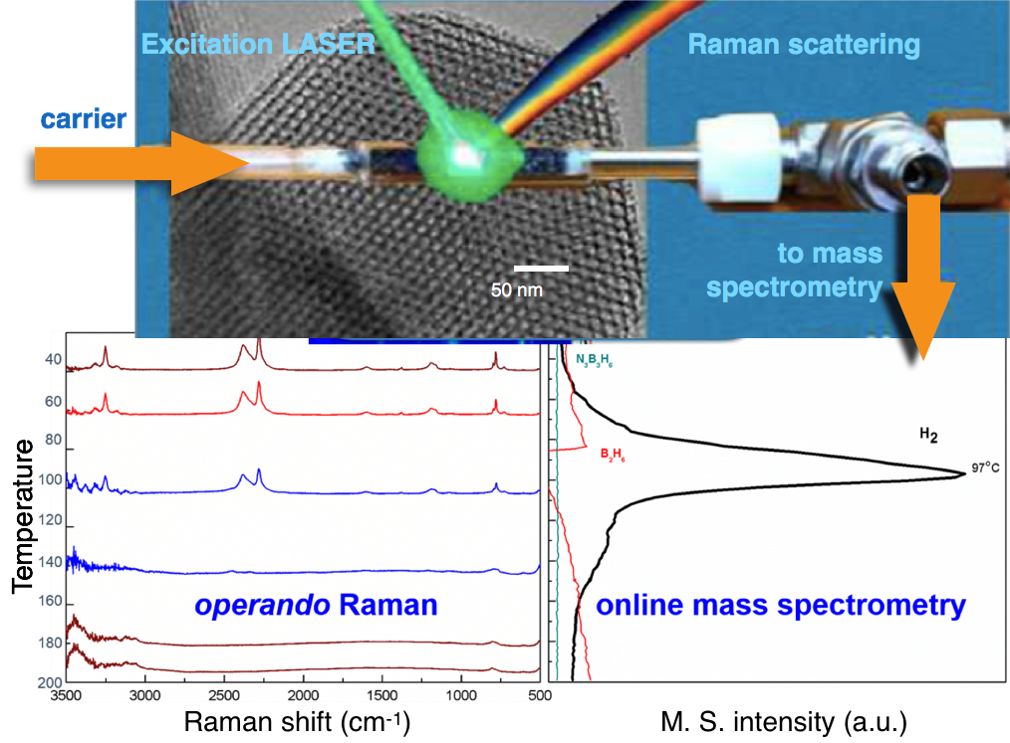
Fig. 1 Simultaneous acquisition of Raman spectra and Mass spectrometry profiles for gases evolved during thermal decomposition of AB impregnated in porous SBA-15 materials.
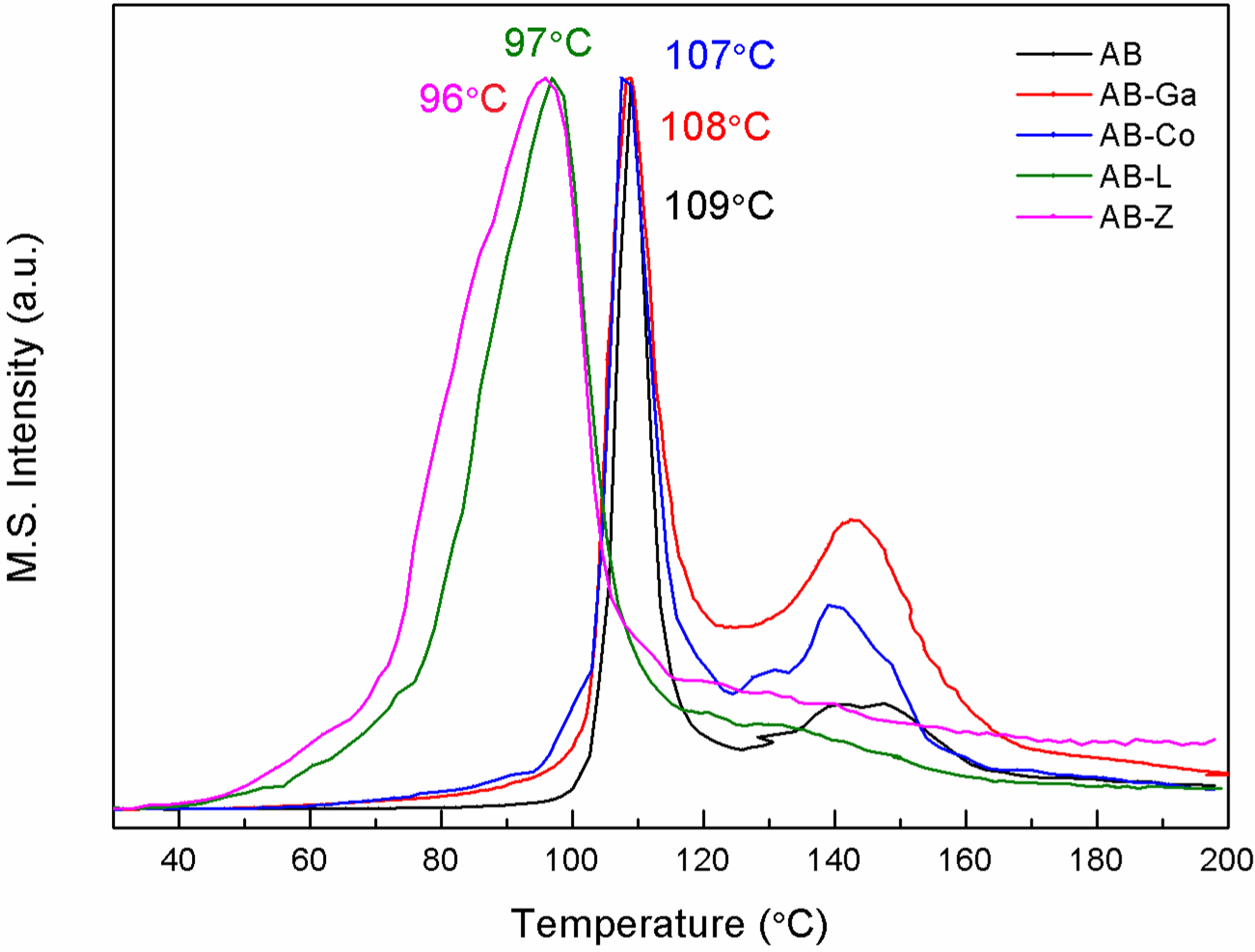
Fig. 2 Comparison of hydrogen mass profile acquired during the heating of neat and impregnated AB under a ramp of 1ºC·min-1.
The MS spectra for neat AB shows that hydrogen release occurs in two stages (Figure 2), in agreement with the two-steps mechanism proposed in the literature [1] for AB thermal decomposition: a first decomposition peak near 109ºC and a second hydrogen release peak near
144ºC are observed along with concomitant release of borazine. Changes in the Raman signals of neat AB suggest the formation of polymeric aminoborane ((-[BH2NH2]n-), PAB) as hydrogen evolution reaches maximum values around 109ºC (Figure 2). The most apparent change in the 140-200ºC temperature range is at the broad 2379 cm-1 B-H stretching mode, which is characteristic of B-H bonds in polyiminoborane ((-[BHNH]n-), PIB).
Hydrogen thermal desorption from AB impregnated into the porous carriers confirms that nanoconfinement facilitates hydrogen release during thermolysis, minimizing undesired BNHx fragments. Furthermore, the comparison of the different pore structures shows that nanoconfinement is critical for such a change.
The influence of CoAPO-5 and mesoporous gallium oxide on hydrogen release is less apparent than that of SBA-15 materials. Like in the case of neat AB, the release takes place through two main events (Figure 2). CoAPO-5 shows a modest intercrystalline mesoporosity combined with microporosity given by a monodimensional channel array. Gallium oxide exhibits some mesoporosity, but its total pore volume is very limited. Thus, these two materials hardly exhibit capacity to host AB. The only difference with neat AB thermal decomposition relies is shown in the onset temperatures of hydrogen release which is already appreciable at temperatures as low as 60ºC (Figure 2). This can be attributed to the destabilization of the small fraction of AB that is nanoconfined within the pores of these hosts. Additionally, borazine release decreases to levels that are not detectable by the mass spectrometer. This effect could be related to a Lewis acid-base interaction of AB with the oxide ions of the hosts.
The effect of nanoconfinement on AB thermolysis is much more pronounced in SBA-15 samples. In contrast with the results obtained with the previous host materials, scaffolding AB into SBA-15 porous materials drastically decrease hydrogen desorption temperatures, with an onset of hydrogen release temperatures as low as 40ºC and a single hydrogen release peak at temperatures significantly lower than those observed with neat AB (Figures 1 and 2). On the other hand, the evolution of Raman spectra during thermolysis indicates a variation in the mechanism of decomposition of AB that can be attributed to chemical interactions with active surface hydroxyl groups in SBA-15 hosts (Figure 1). As suggested in the literature [3], these groups can interact with the BH3 group, weakening the covalent bond between BH3 and NH3 groups of AB, thus destabilizing and promoting the decomposition of the compound. Furthermore, by this interaction BH3 is kept bounded to the scaffold, reducing the production of borazine, and precluding the formation of polyiminoborane as observed in operando Raman-MS experiments.
Confinement of AB within nanoporous hosts can then be enhanced by the chemical interactions between the hydride and the host. According to these results, it can be expected that an appropriate combination of nanoconfinement and chemical environment would facilitate a cleaner hydrogen release by thermolysis of AB.
Microporous and Mesoporous Materials 2016, 226, 454-465 DOI: https://doi.org/10.1016/j.micromeso.2016.02.013
Download Project Summary: AP1242
Project summary by: Maria J. Valero-Pedraza | Espectroscopia y catálisis industrial, Instituto de Catálisis y Petroleoquímica, CSIC, C/ Marie Curie 2, Cantoblanco, 28049, Madrid, Spain
To find out more about this product visit the HPR-20 product page or if you would like to contact us directly please Send us a Message.

