Fischer-Tropsch synthesis (FTS) is a process used to synthesize ultra-clean diesel from crude-oil alternative feedstocks, such as biomass, municipal waste and natural gas. The application of FTS in the chemical industry is experiencing a resurgence, due to increased environmental awareness, an ever-growing demand for (increasingly cleaner) transportation fuels, and the environmental impact from burning fossil carbon. FTS converts synthesis gas, a mixture of CO + H2, over a catalyst to produce the heavier hydrocarbons. An industrially relevant catalyst for this process is in the form of cobalt nanoparticles supported on titania. It is known that this catalyst slowly deactivates but many questions remain regarding details of the deactivation mechanisms.
In this study, a collaboration between Prof. Bert Weckhuysen (Utrecht University) and Simon R Bare (SLAC National Accelerator Laboratory), we examined possible deactivation mechanisms of the Co/TiO2 catalyst over the course of one-week continuous operation under FTS conditions of CO:H2 1:1, 220oC, 16 bar pressure. The intent was not only to provide insight into the deactivation mechanism, but also to illustrate methods to study atomic-level details of long-term deactivation. Over this time the cobalt nanoparticles transformed into cobalt carbide nanoparticles, as determined by both X-ray absorption spectroscopy (XAS) at Stanford Synchrotron Radiation Lightsource (SSRL) at SLAC National Accelerator Laboratory, and XRD in the laboratory at Utrecht University. At SSRL we used a Hiden HPR-20 to detect the gas phase products from the catalysis. It was determined that the product formation does not noticeably change when cobalt carbide formation is detected, suggesting that cobalt carbide formation is not a major deactivation mechanism for this catalyst.
This study shows that it is feasible to conduct longer term catalyst deactivation experiments at a synchrotron facility. Indeed, as a result of the success of this pilot experiment, we are planning on making this capability available to catalyst researchers and use the learnings from this study to develop a user-friendly capability at SSRL.
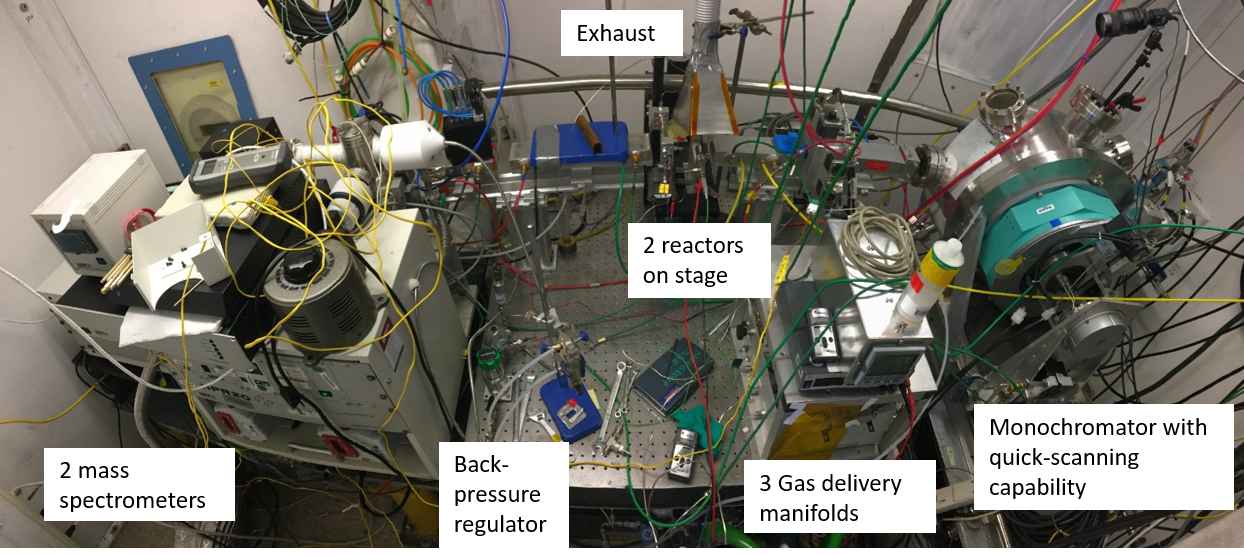
The instrumentation inside the hutch at beamline 2-2 at SSRL for the operando XAS study
The authors thank Shell and the Netherlands Research Council (NWO) for a TA-CHIPP grant. Shell is also thanked for the catalytic testing experiments. Further financial support for this work came from the Netherlands Center for Multiscale Catalytic Energy Conversion (MCEC), an NWO Gravitation program funded by the Ministry of Education, Culture and Science of the government of the Netherlands. Part of this work was also performed at Stanford Synchrotron Radiation Lightsource (SSRL) of SLAC National Accelerator Laboratory and use of the SSRL is supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515 and by Co-ACCESS supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division.
 Project summary by: Simon Bare, SLAC National Accelerator Lab, 2575 Sand Hill Road, Menlo Park CA 94025 USA
Project summary by: Simon Bare, SLAC National Accelerator Lab, 2575 Sand Hill Road, Menlo Park CA 94025 USA
Paper Reference: ACS Catalysis 2021 (11) 2956−2967
Hiden Product: HPR-20
Project Summary Reference: AP-HPR-20-202143_Project Summary
To find out more about these products visit the HPR-20 product page or if you would like to contact us directly please Send us a Message.

