Fast charging of present-day graphite anodes in lithium-ion batteries (LIBs) is essential to effectively decrease the charging time of electric vehicles (EVs), hence, reducing the usage of non-renewable energies and their emissions. However, Li dendrite formation and growth is the key obstacle in the path of fast charging, which affects the cell performance. This is due to a triggering of side reactions such as excessive electrolyte decomposition, thickening electrode/electrolyte interphase (solid electrolyte interphase – SEI), increased resistance to Li+ transportation. To help mitigate these phenomena, an inorganic salt KPF6 has been selected as an electrolyte additive to control Li dendritic growth.
In this work, modified electrolytes were formulated using various concentrations of KPF6 and cycled with graphite | NMC 622 full cells at different C-rates (C/5 to 3C). Among all the concentrations, 0.001M KPF6 is proven to be effective in inhibiting Li dendrite growth at 2C charging rate in contrast to commercial electrolyte (E-0M). It is observed that 0.001M KPF6 additive based electrolyte shows superior coulombic efficiency (CE) at 2C rate, indicating a stabilised SEI layer and resulting in reduced parasitic reaction rates and irreversible capacity loss. X-ray Photoelectron Spectroscopy (XPS) performed on graphite anode cycled optimised electrolyte (0.001M KPF6) shows the presence of a thin SEI film. A higher percentage concentration of metal fluoride in the SEI layer is detected in the case of the optimised electrolyte compared to the commercial one. Secondary Ion Mass Spectroscopy (SIMS) was carried out to identify the ion fragments corresponding to the metal fluoride present in SEI layer.
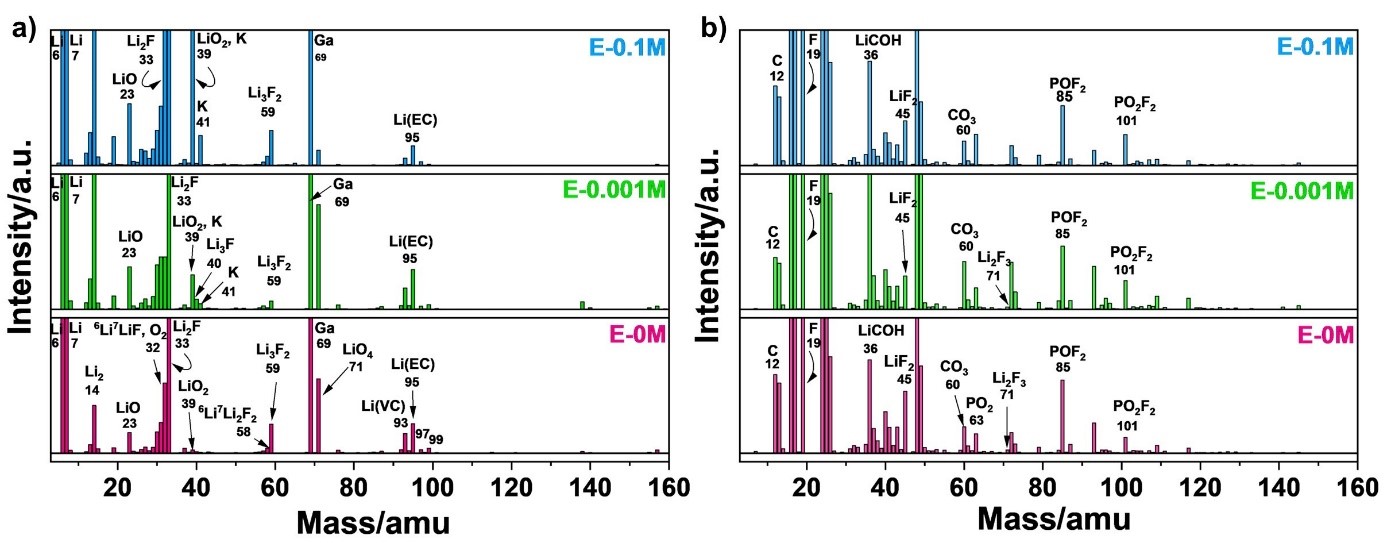
Figure 1. SIMS (a) positive ion and (b) negative ion mode mass spectra of graphite anode surface cycled with E-0M (commercial electrolyte), E-0.001M (optimised electrolyte), E-0.1M (KPF6 additive = 0.1M) electrolytes.
The composition of metal fluoride could be LiF or a combination of LiF and KF, as the optimised electrolyte contains LiPF6 as prime salt and KPF6 as electrolyte additive. Figure 1 represents the positive and negative mode mass spectra, which demonstrates the respective positive and negative ion fragments. The data indicates that the composition of metal fluoride concentration is LiF. High LiF content provides stability and robustness by blocking electron leakage pathway in the SEI layer due to its insulation properties (~ 10-13 to 10-14 S/cm). This facilitates faster Li+ transportation in optimised concentration compared commercial electrolyte. In addition, larger sized K+ ion having faster diffusion rate and lower de-solvation energy (compared to Li+) deposits at the defects sites such as particle boundaries and cracks, prevents the incoming Li+ that could contribute to dendritic growth. This dual effect i.e., K+ occupying the defect sites and LiF blocking electron leakage pathways inhibit the respective Li+ and electrons required for reduction of Li+ to Li0 (metallic Li), enabling a dendritic-free fast-charged graphite anode.

Figure 2. Dualbeam FEG SEM/FIB (Field Emission Gun Secondary Electron Microscopy/ Focused Ion Beam) system.
 Project summary by: Melanie Loveridge, Warwick Manufacturing Group (WMG), University of Warwick, Coventry CV4 7AL, UK
Project summary by: Melanie Loveridge, Warwick Manufacturing Group (WMG), University of Warwick, Coventry CV4 7AL, UK
Paper Reference: “Controlling Li Dendritic Growth in Graphite Anodes by Potassium Electrolyte Additives for Li-Ion Batteries” ACS Applied Material Interfaces (2022) 14, 37, 42078-42092 DOI: 10.1021/acsami.2c11175
Hiden Product: EQS
Download PDF: AP-EQS-202004
To find out more about these products visit the EQS product page or if you would like to contact us directly please Send us a Message.

