The failure of lithium-ion batteries can be partly defined by the release of toxic and flammable gases. The composition and volume of these gases varies depending on cell type, failure mechanism, state of charge (SoC) and environmental oxygen levels. Understanding this relationship and the species produced is essential for battery pack manufacturers relying on real-time monitoring to assess the state-of-health of a LIB. It also has ramifications for emergency responders attending thermal runaway events.
However, a literature review investigating gas analysis of lithium-ion cells has identified several significant gaps. In particular, it identified a variety of experimental set-ups being used, each possessing different fixed volumes or air flows. These factors directly affect the oxygen availability, which in turn influences the ratio of combustion:incomplete combustion, and ultimately, the gas composition. Furthermore, many of these studies acquire gas samples after cell failure has occurred, yet it is evident that the gas composition differs between the initial safety vent through to thermal runaway. As such, real-time gas analysis becomes a powerful technique to probe the behaviour and composition of battery vent gas.
Test Campaign Summary
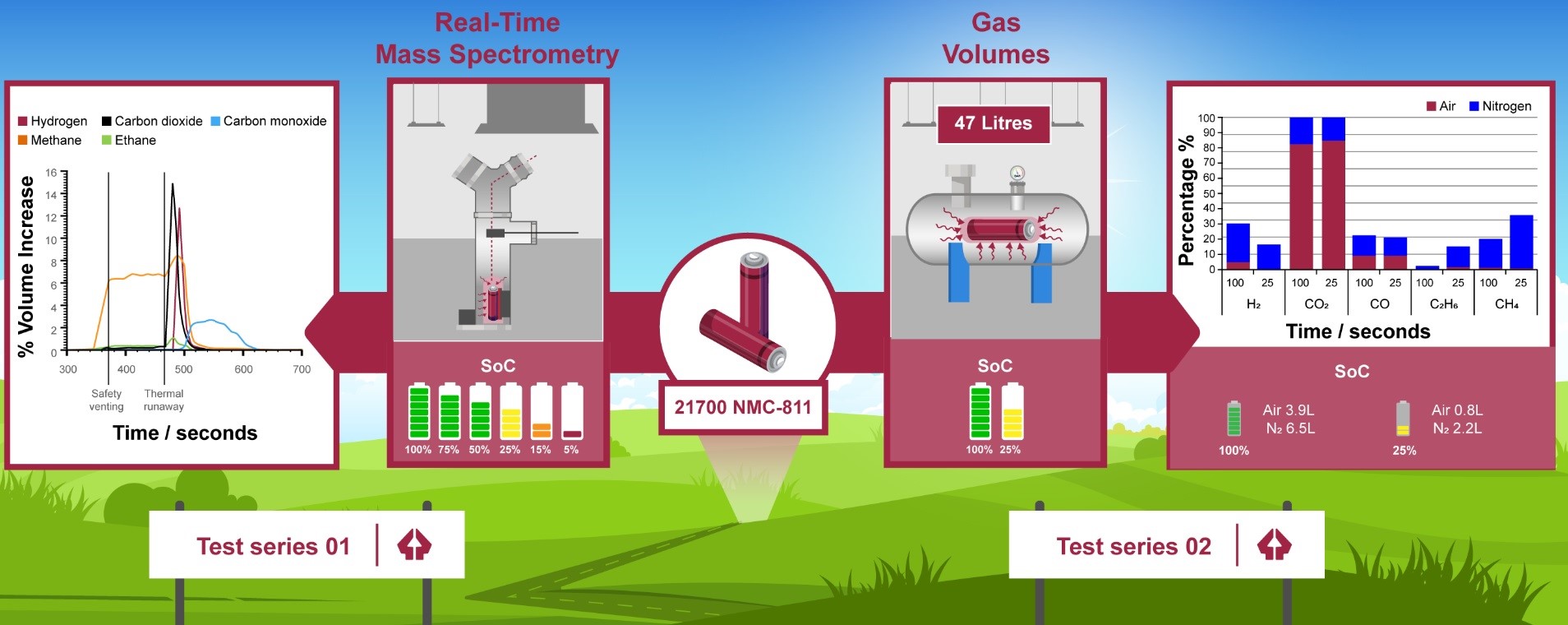
In this study, we used a Hiden HPR-20 mass spectrometer to analyse, in real-time, the battery vent gas released from a commercially available NMC-811 21700 cell. The NMC chemistry was selected based on its prevalence in many high performing applications, with a view that Ni-enriched cells will remain in high demand due to high cost and sustainability issues associated with cobalt. In our experimental set-up, the MS was placed in-line with a custom-built rig to investigate gases released at different SoC. The cell article was heated using an external, adhesive heater until after thermal runaway had occurred.
The real-time results showed that the gas composition during safety venting was mostly methane, and that the methane concentration was positively correlated with SoC. Furthermore, we used mass spectrometry to monitor for signs of electrolyte release; this was confirmed through positive signals at m/z 90 and m/z 77 for dimethyl carbonate and ethyl methyl carbonate respectively.
During thermal runaway, we noted a distinct shift in the overall composition. We observed a rapid rise in hydrogen, carbon dioxide and carbon monoxide. Furthermore, hydrogen was present in similar concentrations to carbon dioxide, which raises questions regarding the flammability of such mixtures.
Finally, to verify our results, we performed tests using a 50 L pressure vessel to determine gas volumes and total gas compositions in both air and nitrogen. At the end of each test, the gas was collected using a Tedlar sampling bag and analysed using the HPR-20. Through this, we showed that, at 100% SoC, hydrogen and carbon dioxide accounted for more than 50% of 6.5 L of gas produced. At 25% SoC, however, methane and carbon dioxide were the two primary constituent gases.
In summary, we demonstrated that battery monitoring systems should consider VOC sensors as a potential pre-warning measure. Furthermore, we have identified that the battery vent gas contains multiple flammable components and further research must now be undertaken to determine flammability limits.
Project summary by: Daniel Howard, HSE Science and Research Centre, Harpur Hill, Buxton, Derbyshire, SK17 9JN, United Kingdom
Paper Reference: “Comprehensive gas analysis of a 21700 Li(Ni0.8Co0.1Mn0.1O2) cell using mass spectrometry”, Journal of Power Sources, 539 (2022) 231585 DOI: 10.1016/j.jpowsour.2022.231585
Hiden Product: HPR-20
Download PDF: AP-HPR-20-202377
To find out more about these products visit the HPR-20 product page or if you would like to contact us directly please Send us a Message.

