The importance of investigating the acidity of heterogeneous catalysts cannot be overstated, particularly when acidity is the key factor ruling the outcome of a reaction. Wrong acidic features can easily lead to undesired products and/or, in the worst cases, to the deactivation of the catalyst. Both the number of the acid sites and their strength are therefore crucial and ammonia temperature programmed desorption is arguably one of the most used techniques to investigate them. This analysis basically consists in adsorbing gaseous ammonia on the catalyst at a certain temperature followed by its desorption by ramping up the temperature at a desired velocity. To calculate the amount of ammonia desorbed—which can be correlated to the number of acid sites—we utilized a home-made apparatus that allowed us to send to the catalyst a desired amount of both ammonia and carrier gas, connected downstream to a Hiden HPR-20 quadrupole mass spectrometer.
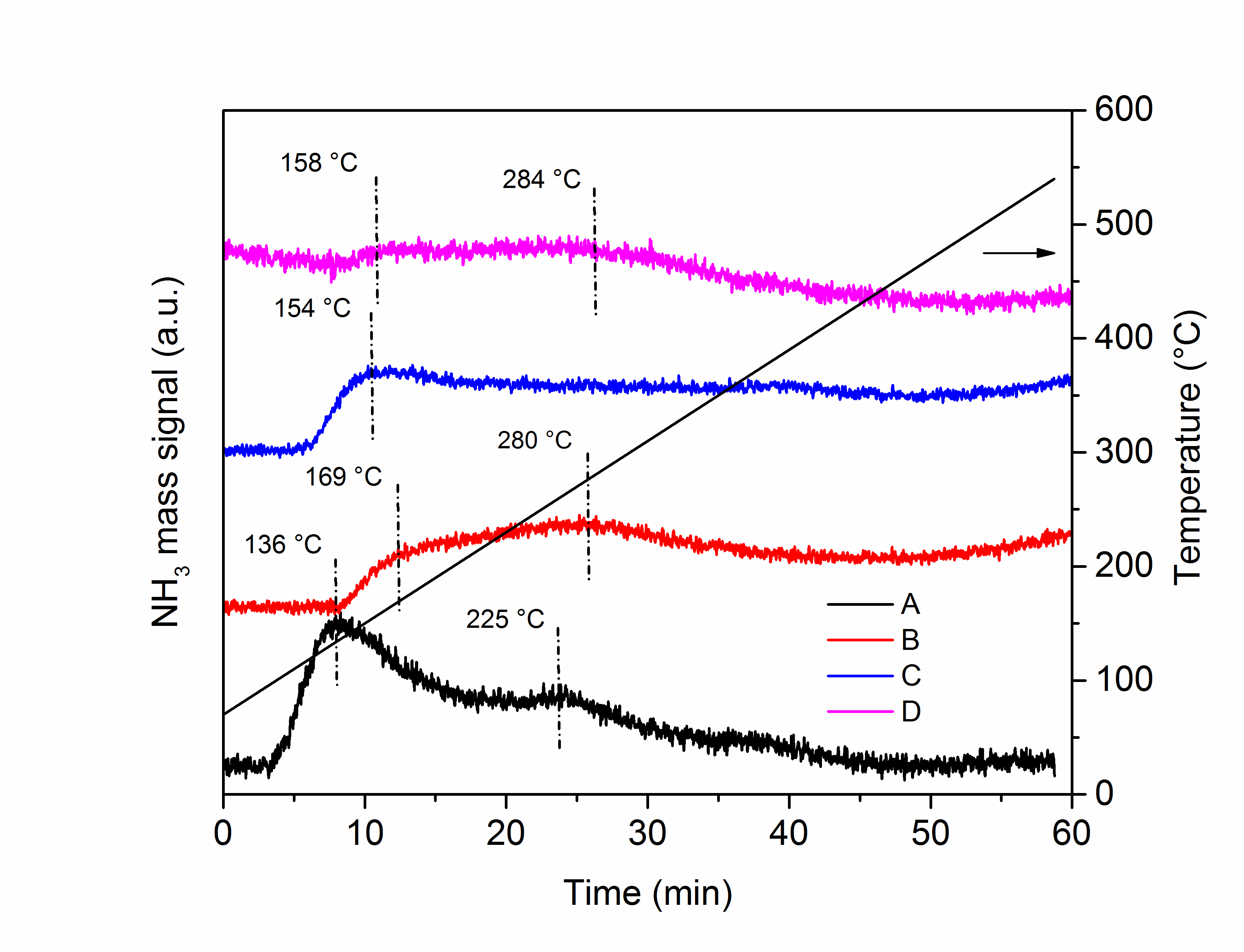
Figure 1. Ammonia Temperature Programmed Desorption (NH3-TPD) profiles of the catalysts.
We monitored the main mass signal of ammonia as a function of time—and in turn temperature. From these analyses we found that the choice of the metal precursors has a major role in determining both the total number of acid sites, calculated from the area under the ammonia desorption profiles, and, more importantly, the strength of such acid sites, as differences in the desorption peak positions. Most interestingly, the catalyst that showed the highest activity in the cinnamaldehyde hydrogenation reaction among those investigated was the one with the strongest sites and not the highest number (catalyst B in the figure). This means that sometimes it is not the sheer number of acid sites that makes the difference but, rather, their strength. Quality over quantity.
Paper Reference: “Ruling Factors in Cinnamaldehyde Hydrogenation: Activity and Selectivity of Pt-Mo Catalysts” Nanomaterials 2021, 11, 362 https://doi.org/10.3390/nano11020362
Download Project Summary: AP-HPR-20-202129
 Project summary by: Filippo Bossola, Istituto di scienze e tecnologie chimiche “Giulio Natta” (SCITEC) CNR, Via C. Golgi 19, 20133 Milano, Italy
Project summary by: Filippo Bossola, Istituto di scienze e tecnologie chimiche “Giulio Natta” (SCITEC) CNR, Via C. Golgi 19, 20133 Milano, Italy
To find out more about this product visit the HPR-20 product page or if you would like to contact us directly please Send us a Message.

