Authors: Marek Rotko, Andrzej Machocki
The catalytic process of complete methane oxidation is a highly promising alternative to flame combustion, because it makes it possible to reduce the emission of NOX, CO and non-oxidized hydrocarbons into the Earth’s atmosphere. Some of the most active catalytic materials for complete methane oxidation are supported palladium and platinum catalysts. They demonstrate very high activity and selectivity; also, their resistance to high temperature and mechanical damage is acceptable. However, for the wide application of palladium and platinum catalysts in the industry, there is still a need for clear answers to many important questions. One of the most crucial issues is the reaction mechanism of complete methane oxidation over palladium and platinum catalysts. We studied this problem by means of the SSITKA method (Steady State Isotopic Transient Kinetic Analysis).
The SSITKA method enables obtaining much unique and valuable information concerning mechanisms of various heterogeneous catalytic reactions, as well other equally important issues such as: the number of active centres on the catalyst surface, an average surface life-time and surface concentrations of reagents, intermediates and products of the catalytic reaction. However, the SSITKA study needs very high-quality and precise equipment, in particular, a system of dosing and fast switching of reagent streams as well as a mass spectrometer, which makes it possible to investigate even extremely low changes in the concentration of isotopes. In our experiments, the switches between reaction streams including 12CH4/Ar/O2/He and 13CH4/Kr/O2/He as well as between 16O2/Ar/CH4/He and 18O2/Kr/CH4/He were carried out. The examples of results for the Pd-Pt/Al2O3 catalyst are included in Fig. 1.
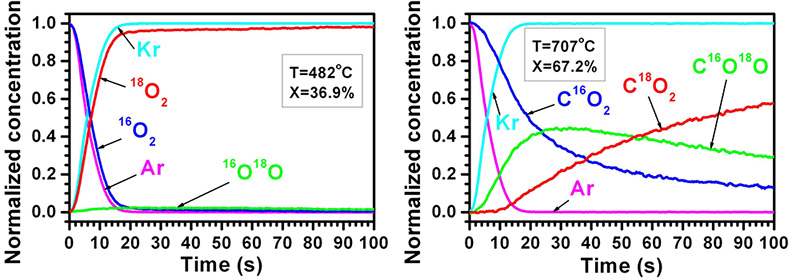
Fig. 1. Effect of the switching between reaction streams including 16O2/Ar/CH4/He and 18O2/Kr/CH4/He (X is the conversion of methane).
On the basis of such results and after a comprehensive analysis (widely described in the reference paper), it has been proposed that two different kinds of active centres (α – more active, but less numerous and β – less active, but more numerous) exist on the catalyst surface and the process of methane oxidation proceeds simultaneously according to two different reaction mechanisms (by Mars-van Krevelen and Langmuir-Hinshelwood). The level of their participation in methane oxidation is different and depends on the reaction temperature. What is more, the process of methane oxidation proceeds not only simultaneously, according to two different reaction mechanisms, but also with different reaction rates determined by the type of active centres.
Project summary by:
 Marek Rotko, Andrzej Machocki
Marek Rotko, Andrzej Machocki
University of Maria Curie-Skłodowska
Faculty of Chemistry
Department of Chemical Technology
3 Maria Curie-Skłodowska Square
20-031 Lublin
Poland
Paper Reference:
- Rotko, A. Machocki, G. Słowik (2014) “The mechanism of the CH4/O2 reaction on the Pd-Pt/γ-Al2O3 catalyst: A SSITKA study” Applied Catalysis B: Environmental, 160-161 298-306

