![]() Differential electrochemical mass spectrometry (DEMS) has been extensively used for studying fuel cell related electrocatalytic reactions and proven to be crucial for deriving molecular level insight for such processes. It has a great advantage in not only qualitative but also quantitative analysis such as products rate and current efficiencies of various products from complex reactions. For all quantitative studies, precise calibration of the mass signal is a prerequisite. In this work, we report the effect of alcohol concentration on the detected DEMS signal of CO2, a common final product from electrochemical oxidation of small organic molecules. Comparing to the solution with low alcohol concentration and the same CO2 concentration, a significant reduction of mass signal of CO2 in highly concentrated methanol and ethanol solution (>0.1 M) were observed. Such results reveal that in solutions with high alcohol concentration, alcohol molecules will significantly affect the mass calibration constant for CO2. When the alcohol concentration is above 0.1 M, it is inappropriate to use the mass calibration constant derived from CO oxidation in alcohol free solution as usually done in the literature.
Differential electrochemical mass spectrometry (DEMS) has been extensively used for studying fuel cell related electrocatalytic reactions and proven to be crucial for deriving molecular level insight for such processes. It has a great advantage in not only qualitative but also quantitative analysis such as products rate and current efficiencies of various products from complex reactions. For all quantitative studies, precise calibration of the mass signal is a prerequisite. In this work, we report the effect of alcohol concentration on the detected DEMS signal of CO2, a common final product from electrochemical oxidation of small organic molecules. Comparing to the solution with low alcohol concentration and the same CO2 concentration, a significant reduction of mass signal of CO2 in highly concentrated methanol and ethanol solution (>0.1 M) were observed. Such results reveal that in solutions with high alcohol concentration, alcohol molecules will significantly affect the mass calibration constant for CO2. When the alcohol concentration is above 0.1 M, it is inappropriate to use the mass calibration constant derived from CO oxidation in alcohol free solution as usually done in the literature.
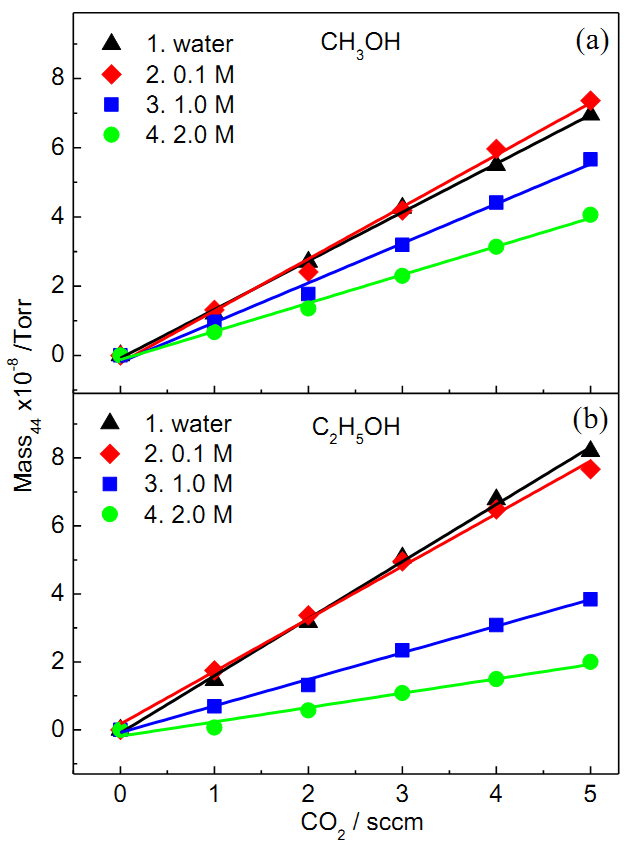
Fig. To simulate the solution containing various amount of CO2 produced during electrocatalytic oxidation of alcohols, the solution is purged with a gas mixture of N2:CO2=500 sccm: 0~5 sccm in :1) pure water (triangle), 2) 0.1 M (diamond), 3) 1 M (square) and 4) 2 M (circle) (a) CH3OH or (b) C2H5OH as a function of CO2 flow rate in the N2+CO2 gas.
Project summary by:
Wei Chen, Qian Tao, Jun Cai and Yan-Xia Chen
Hefei National Laboratory for Physical Sciences at Microscale
Department of Chemical Physics,
University of Science and Technology of China
Hefei, 230026
China
Paper Reference:
Wei Chen, Qian Tao, Jun Cai and Yan-Xia Chen (2014) “The effect of alcohol concentration on the mass signal of CO2 detected by differential mass spectrometry” Electrochemistry Communications 48, 10-12

