In this study, LaSrMnO3 and LaCaMnO3 based perovskite families were primarily doped with A-earth and B-earth elements and composition optimization was achieved with the focus of hydrogen production capacities. For this purpose, La1-xSrxMnyAl1-yO3-SLMA (Sr=0.4-0.6, Al=0.4-0.6) and La1-xCaxMnyAl1-yO3-LCMA (Ca=0.4-0.8, Al=0.4-0.8), LCMF (Ca=0.4) A total of 36 different perovskite oxides containing compositions of Fe=0.4-0.8 and LCMC (Ca=0.4-0.8, Co=0.4-0.8) were synthesized and these compositions were evaluated using thermochemical redox reactions (Tred=~1400 °C, Tox=~800 °C).
In the study oxides that provide relatively high hydrogen production, were determined as candidate compositions. In order to examine the effect of hetero-interface formation on hydrogen production with these candidate compositions, 27 different LSMA:LCMA, LSMA:LCMF and LSMA:LCMC dual-perovskites were produced in composite structure. The produced composite dual-perovskites were also evaluated according to their hydrogen production capacity in two-step thermochemical redox cycles by mass spectrometry, Hiden Analytical QGA.
With this approach, it was aimed to obtain relatively high hydrogen production capacities with a composite catalyst consisting of two different perovskite oxides. In addition to the hydrogen production capacity, another important point in the dual-perovskite approach is that the hydrogen production capacities to be obtained can be stably maintained after redox cycles. It is known in the literature that the hydrogen production capacity of perovskite oxides decreases significantly (<40%) after a few cycles. With this project, perovskite itself and dual combinations were evaluated in terms of hydrogen production capacities.
This study was carried out under the project titled “Development of Perovskite Oxides for Two-Step Thermochemical Water Splitting”, at the Energy Materials Laboratory in the Department of Metallurgical and Materials Engineering at Mugla Sitki Kocman University, with the support of the Scientific and Technological Research Council of Turkey (TÜBİTAK) (Project No: 119M420).
Development of Perovskite Oxides for Two-Step Thermochemical Water Splitting
 Project summary by: Assist. Prof. Dr. Berke PİŞKİN, Metallurgical and Materials Engineering, Mugla Sitki Kocman University, with the support of the Scientific and Technological Research Council of Turkey (TÜBİTAK), 48000 Mugla, TURKEY
Project summary by: Assist. Prof. Dr. Berke PİŞKİN, Metallurgical and Materials Engineering, Mugla Sitki Kocman University, with the support of the Scientific and Technological Research Council of Turkey (TÜBİTAK), 48000 Mugla, TURKEY
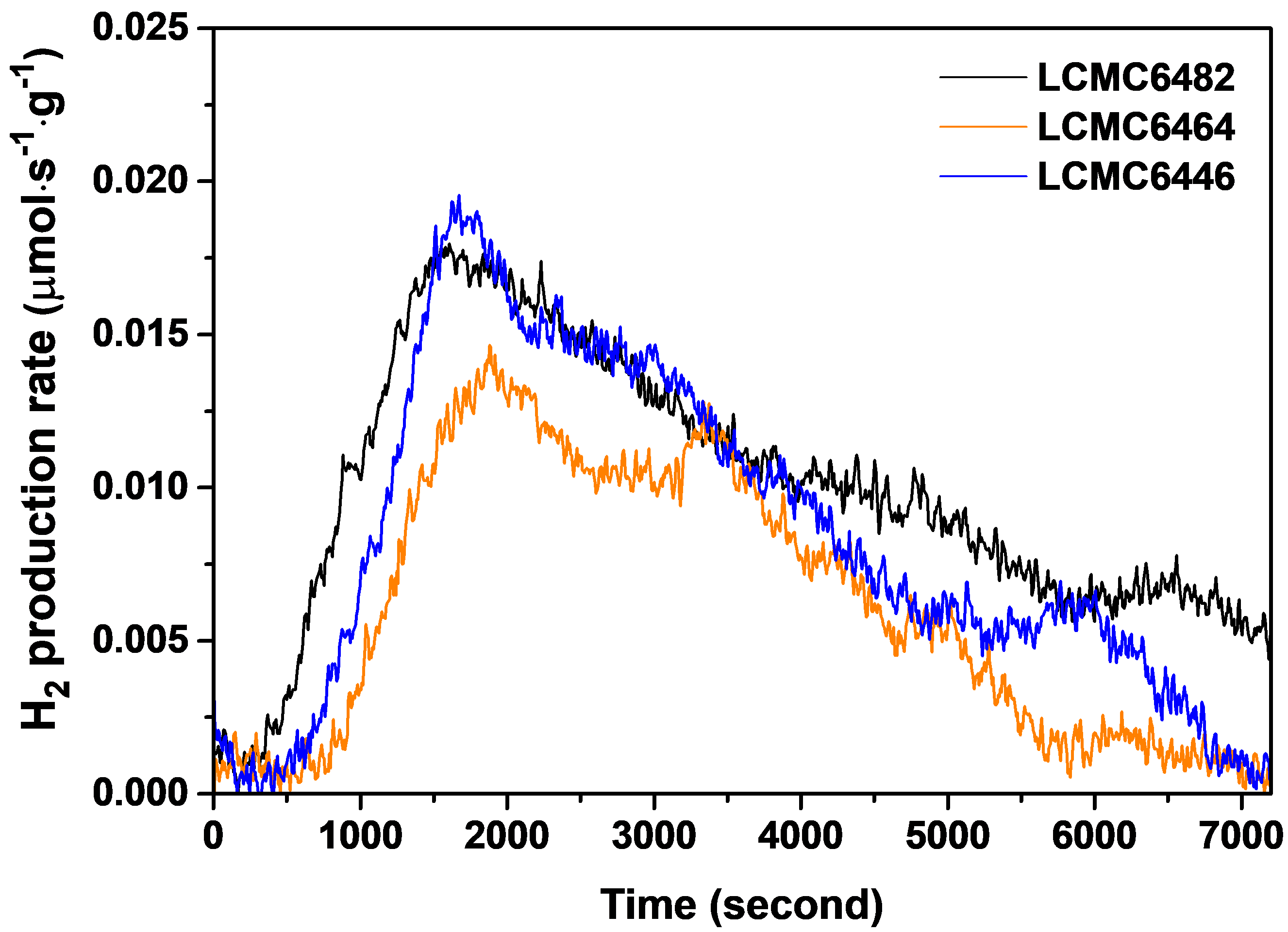
Paper Reference: “Investigation into Ca-Doped LaMnCoO3 Perovskite Oxides for Thermochemical Water Splitting”, JOM 74, 4682-4694 (2022) DOI: 10.1007/s11837-022-05493-9
Hiden Product: QGA
Download PDF: AP-QGA-202279
To find out more about these products visit the QGA product page or if you would like to contact us directly please Send us a Message.

