MgO-based sorbents have received a great attention as a sorbent for pre-combustion CO2 capture. Still, few studies have been conducted on how the sorption mechanism varies in the presence of gases other than CO2. Here, we report on the dynamic CO2 sorption behavior of MgO-based sorbents under simulated practical conditions, including H2O and CO.
The CO2 sorption behavior of the salt-promoted MgCeOx prepared by a sol-gel combustion-assisted method was examined in the fixed bed reactor by monitoring the breakthrough of effluent gases (CO2, CO, Ar) with an on-line mass spectrometer (Hiden HPR-20). The breakthrough experiments under various practical relevant conditions indicated that the feed gas composition was a dominant factor in determining the dynamic CO2 sorption behavior (Figure 1), whereas the breakthrough profiles was not significantly influenced by the sorption temperature conditions. When H2O was included up to 60% in the feed gas (wet condition), the breakthrough time increased significantly from 159 sec to 213 sec compared to the dry condition (Figure 1(a)). Even under the wet condition, the presence of CO in the feed condition resulted in the immediate breakthrough of a trace amount of CO2 and decreased the CO2 sorption capacity of the sorbents (Figure 1(a)). Based on the understanding of dynamic CO2 sorption behavior from the breakthrough experiment, the underlying sorption mechanisms influenced by H2O and CO molecules were studied via in situ DRIFTS analyses under various CO2 mixtures.
In in situ DRIFTS (Diffuse Reflectance Infrared Fourier-Transform Spectroscopy) analyses where the change of OH groups generated by the presence of H2O was observed, the peak intensities of multi-coordinated and mono-coordinated OH groups in the wet conditions increased by 2.32 and 1.72 times, respectively, compared with those in the dry conditions. These results indicated that the surface OH groups were developed by the dissociation of H2O at the oxygen vacancies on the MgCeOx surface, which were subsequently occupied by the OH groups (Figure 2(a)). In particular, compared with other surface species, the peak intensities of the adsorbed OH groups and monodentate carbonate in wet conditions increased at remarkably high rates. The results suggested that H2O may function as a crucial component in the initial rapid sorption of CO2, allowing CO2 to be bound in the form of monodentate carbonate.
 To understand the immediate breakthrough of CO2 in the presence of CO, the CO2 pulse titration experiments were conducted by exposing the MgCeOx sorbent to the dilute CO flow, purging with the inert gas, and analyzing generated gases with the mass spectrometer during the consecutive CO2 pulses. The result revealed that the interrupted CO2 sorption in the co-existence of CO and H2O was caused by competitive sorption (Figure 1(b) and Figure 2(b)). First, the WGS (Water-Gas Shift) reaction was catalyzed by MgCeOx to generate CO2. Then, CO and the WGS-induced CO2 competitively occupied the sorption sites where CO2 in the feed gas could have been sorbed, thereby hindering initial rapid CO2 sorption.
To understand the immediate breakthrough of CO2 in the presence of CO, the CO2 pulse titration experiments were conducted by exposing the MgCeOx sorbent to the dilute CO flow, purging with the inert gas, and analyzing generated gases with the mass spectrometer during the consecutive CO2 pulses. The result revealed that the interrupted CO2 sorption in the co-existence of CO and H2O was caused by competitive sorption (Figure 1(b) and Figure 2(b)). First, the WGS (Water-Gas Shift) reaction was catalyzed by MgCeOx to generate CO2. Then, CO and the WGS-induced CO2 competitively occupied the sorption sites where CO2 in the feed gas could have been sorbed, thereby hindering initial rapid CO2 sorption.
This study showed the approach to understand the dynamic sorption behavior by coupling the result from the breakthrough experiment and the in situ DRIFTS and the obtained result can give a guide to overcome the obstacles for the ultimate application of MgO-based sorbents.
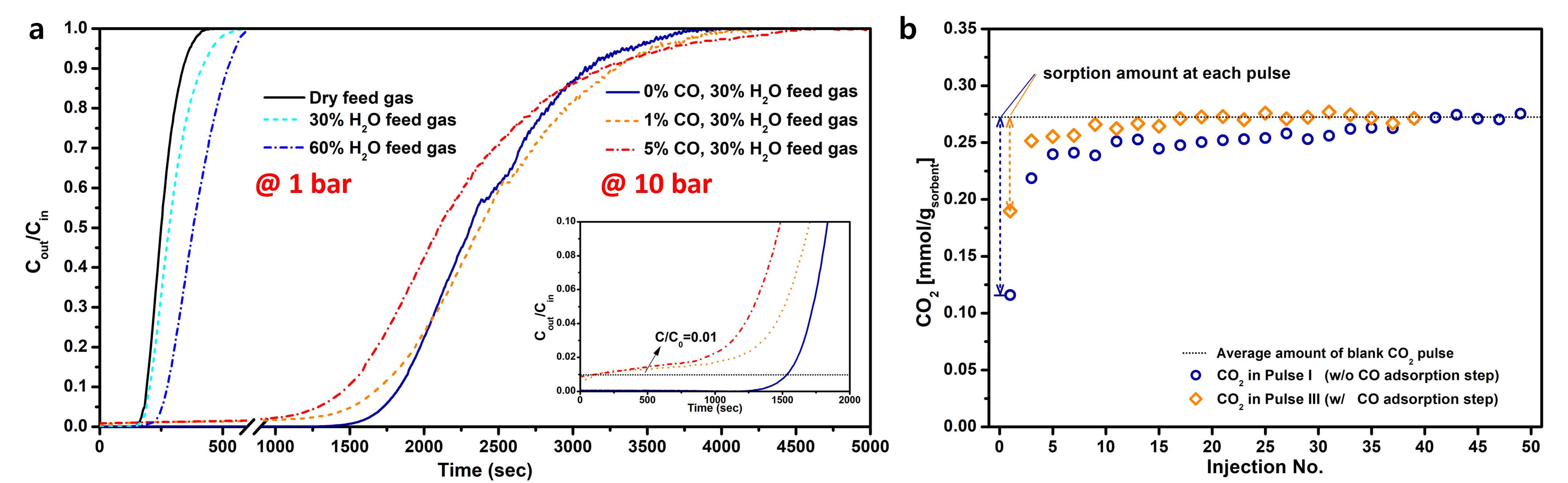
Figure 1. (a) CO2 breakthrough experiments at different pressures under feed mixtures flow including H2O and/or CO and (b) CO2 pulse titration experiments with (Pulse III) and without (Pulse I) CO adsorption step for salt-promoted MgCeOx.
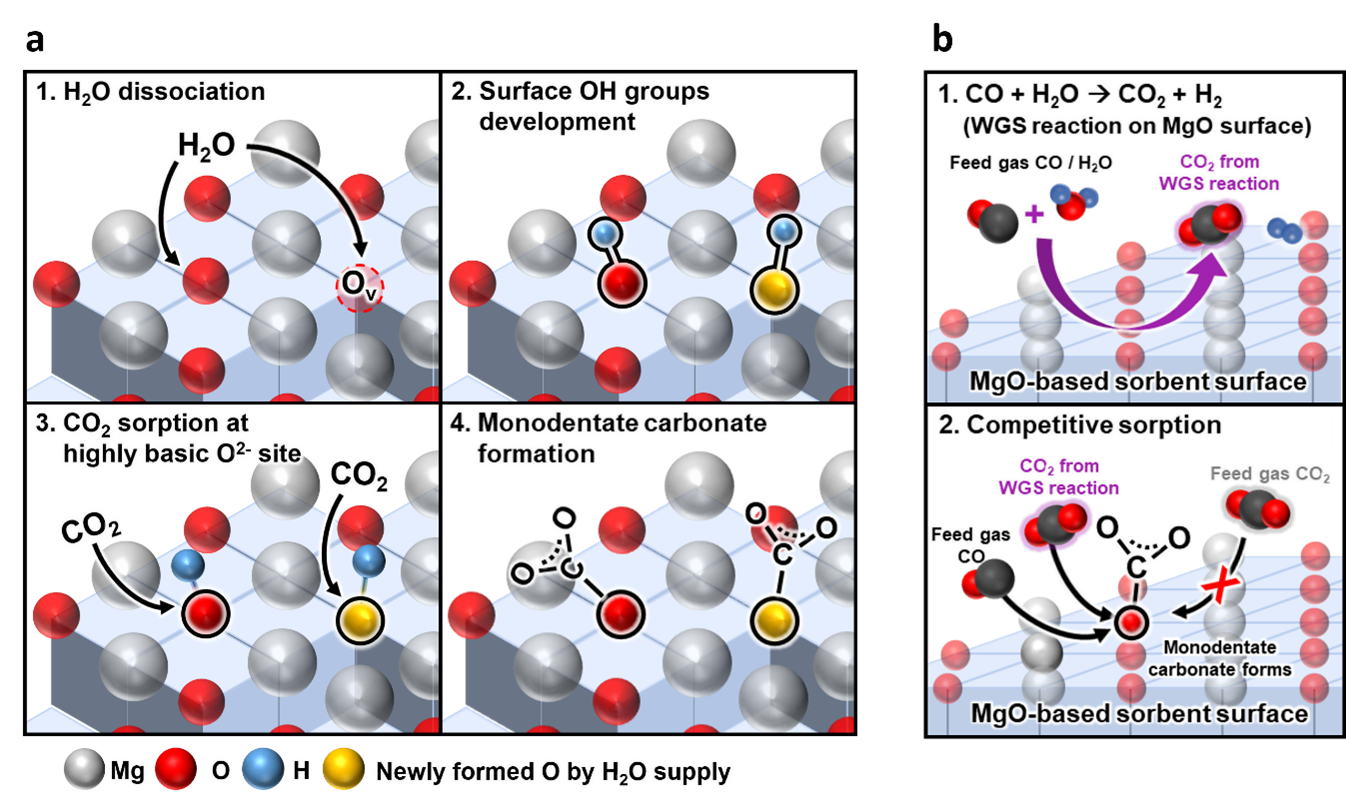
Figure 2. Schematics of CO2 sorption mechanism (a) in wet CO2 condition and (b) in wet CO2/CO condition on the surface of MgO-based sorbent.
 Project summary by: Gina Banga, Seongmin Jina. b and Chang-Ha Leea
Project summary by: Gina Banga, Seongmin Jina. b and Chang-Ha Leea
a Department of Chemical and Biomolecular Engineering, Yonsei University, Seoul, 03722, Republic of Korea
 b Institute of Chemical Engineering, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
b Institute of Chemical Engineering, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland
Paper Reference: Gina Bang, Kyung-Min Kim, Seongmin Jin, Chang-Ha Lee (2022) “Dynamic CO2 sorption on MgO-based sorbent in the presence of CO and H2O at elevated pressures” Chemical Engineering Journal, 433, 134607, DOI: https://doi.org/10.1016/j.cej.2022.134607
Hiden Product: HPR-20
Reference: AP-HPR-20-202267
To find out more about these products visit the HPR-20 product page or if you would like to contact us directly please Send us a Message.

