Quadrupole mass-spectroscopic analysis – in rare gas (M) diluted CH2F2 plasma – has revealed selective formation of CH2F+ ion for Ar dilution and CHF2+ ion for Kr dilution. Ion densities of CH2F+ and CHF2+ were determined by dissociative ionization pathways in channels of charge exchange collisions, i.e., CH2F2 + M+ → CH2F+ + F· + M* and CHF2+ + H· +M* in CH2F2 plasmas. In Ar-diluted plasmas, CH2F+ ions predominated due to dissociative ionization between Ar+ (ca. 15.8 eV) and C-F appearance energy (ca. 16 eV) to form CH2F+. In contrast, for Kr-diluted plasmas, C-H appearance energy (ca. 13.8 eV) predominated to produce a larger amount of CHF2+ ions due to a similar channel for charge exchange collisions between Kr+ (ca. 14 eV) and CH2F2. In accordance with the analytic results, the addition of Ar and Kr gas to CH2F2plasmas provided control over the fraction of CH2F+ and CHF2+ ion densities.
Hydrofluorocarbons have H atoms in place of F atoms in fluorocarbon gases. Dissociation reactions involving C−H and C−F bonds are of interest for controlling the density of reactive species: F atoms, produced by dissociation of the C−F bond, are a main etchant for Si, while H atoms, produced by dissociation of the C−H bond, promote the deposition of polymers on a substrate surface. For processing accuracy, a balance of species for etching and deposition is believed to be important and to be closely related the dissociation processes in gas-phase. However, the variety and densities of the ions and radicals generated in hydrofluorocarbon plasmas have not been fully elucidated.
The experiments was performed with a dual frequency capacitively coupled plasma (CCP) etching reactor, installed a quadrupole mass spectrometer (QMS; Hiden Analytical, EQP) at the chamber wall. A 100-μm diameter aperture was installed in the QMS entrance. A mixture of Ar or Kr gas with CH2F2 gas was introduced into a chamber, and plasmas were sustained by applying the very high frequency (VHF) power to the electrode.
Positive ion mass spectrometric measurements revealed that the dominant positive ions were CH2F+ and CHF2+. In the ionization pathway generated for CH2F+ and CHF2+ ions, two channels are involved: CH2F+ through C-F bond dissociation or through C-H bond dissociation. The reaction schemes for the dissociative reactions in electron collisions are given by CH2F2 + e- → CH2F+ + F + 2e- (threshold at 15.8 eV), and → CHF2+ + H + 2e- (13.8 eV). The counter fragments of charge-neutral H and F atoms were generated simultaneously through these dissociation mechanisms. A larger ion density for CH2F+ in the Ar-diluted plasma, other dissociation processes but the electron collisions need to be considered. Charge exchange collisions between rare gas ions and CH2F2molecules occurred, because the appearance energies were located close to that for Ar (16 eV) and Kr (14 eV).
We concentrate our continuous study in elucidation of the dissociative reactions in plasma through the gas-phase diagnostics utilized the mass-spectrometric measurements.
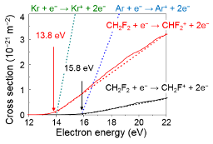
Figure 1: Cross section for dissociative ionization for a CH2F2 molecule
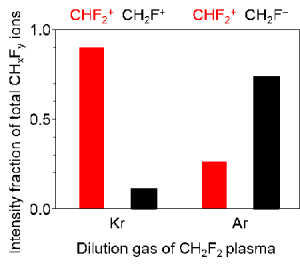
Figure 2: Individual CH2F+ and CHF2+ ion fraction on total CHxFy+ ion density at Kr- and Ar-diluted CH2F2 plasma
Project summary by:
Yusuke Kondo, Kenji Ishikawa, Toshiya Hayashi, and Masaru Hori, et al.,
Nagoya University, Furo-cho, Chikusa, Nagoya 464-8603, JAPAN
Visit Product Page : EQP
View Full Newsletter : Mass Spectrometers for Plasma Characterisation
Make an enquiry : Send us a message

