Adsorbate-induced structural evolution changes the mechanism of CO oxidation on a Rh/Fe3O4(001) model catalyst
Nanoscale 2020, 12, 5866-5875 doi:10.1039/c9nr10087c
The structure of a catalyst often changes in reactive environments, and following the structural evolution is crucial for the identification of the catalyst’s active phase and reaction mechanism. Using TPD combined with other techniques, an atomic-scale study of CO oxidation on a model Rh/Fe3O4(001) “single-atom” catalyst has demonstrated that different changes in catalyst structure and consequently different reactions mechanisms can occur depending on which of the two reactants (O2 or CO) is adsorbed first.
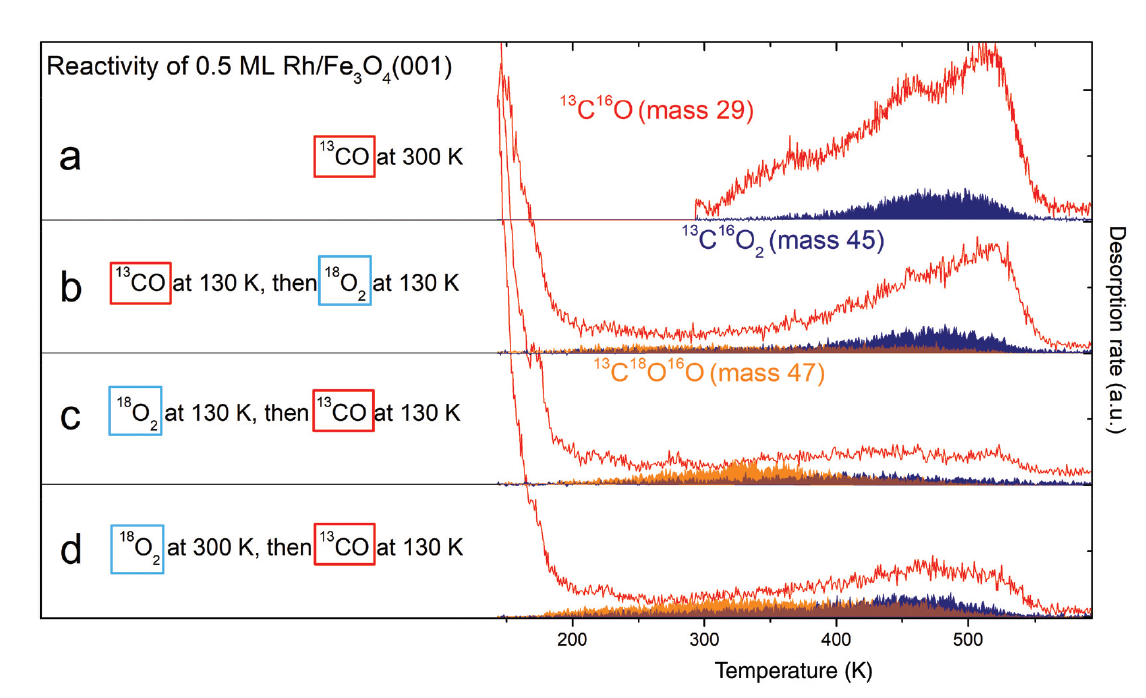
Figure 7. Probing the reactivity of 0.5 ML Rh/Fe3O4(001) by TPD after sequential dosing of 13CO and 18O2. Red traces correspond to 13CO, blue areas to 13C16O2 produced via a MvK mechanism and orange areas correspond to 13C16O18O produced via a L–H mechanism. (a) Dosing 13CO at 300 K results in spectra comparable to Fig. 6a. (b) Dosing 13CO at 130 K, then 18O2 at 130 K leads to a lower 13CO desorption signal, but overall higher 13CO2 production below 400 K. (c) Dosing 18O2 first at 130 K, then 13CO at 130 K leads to significant CO2 signal produced by the L–H mechanism, the MvK channel is suppressed. (d) Dosing 18O2 at 300 K, then 13CO at 130 K leads to similar results as (c), but with more CO2 produced above 400 K in both reaction channels.
You can Download & read the full paper or View it online.
To find out more about this product visit the 3F Series product page or if you would like to contact us directly please Send us a Message.

